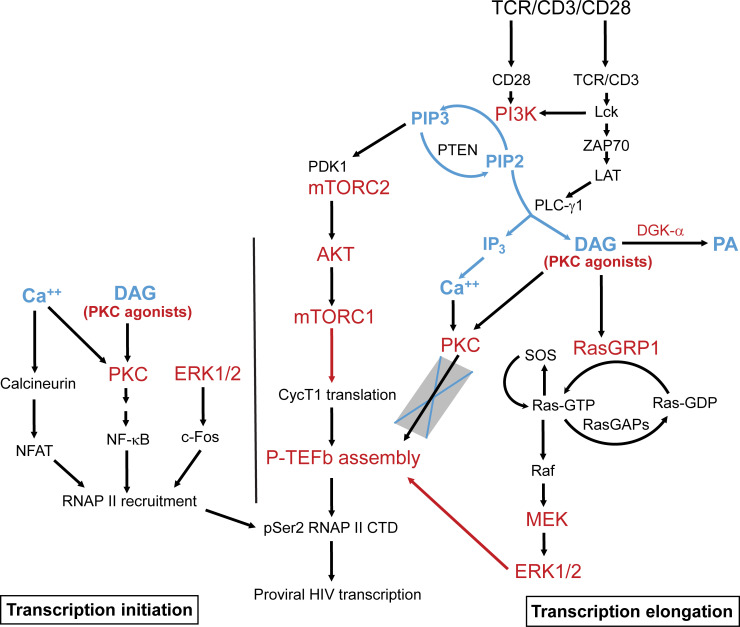
Fig 10. Model for the reactivation of P-TEFb and proviral HIV by RasGRP1-Ras-Raf-MEK-ERK1/2 and PI3K-AKT-mTORC1/2 signaling in latently infected CD4+ T cells.
Generation of diacylglycerol (DAG) by activated PLC-γ1 immediately following TCR co-stimulation enables the recruitment of RasGRP1 to the plasma membrane, the initial activation of Ras via GTP loading and the allosteric activation of membrane-anchored SOS by GTP-bound Ras. This can also be accomplished by DAG-mimicking PKC agonists. Allosteric activation of SOS creates a positive Ras-GTP-SOS feedback loop that leads to maximal activation of Ras and formation of Ras-Raf complexes at the membrane. A phosphorylation cascade is triggered by Ras-Raf leading to activation of ERK1 and ERK2 which in turn stimulates the biogenesis of P-TEFb via mechanisms that are yet to be defined. RasGRP1 activity may be regulated by controlling the availability of DAG which, based on the RNA-seq data shown in S18 Fig, may get converted to phosphatidic acid (PA) primarily by DGK-α in primary T cells. TCR co-stimulation also rapidly stimulates PI3K leading to the activation of mTORC1 which may regulate P-TEFb biogenesis by enabling posttranscriptional synthesis of cyclin T1 (CycT1). Inhibitor-based experiments have clarified that intracellular calcium release and the activation of PKC are dispensable for formation of transcriptionally active P-TEFb. However, the recruitment of RNA polymerase II (RNAP II) to proviral HIV and its eventual phosphorylation by P-TEFb to stimulate processive transcription elongation are likely to be dependent on both Ca++ and PKC signaling.