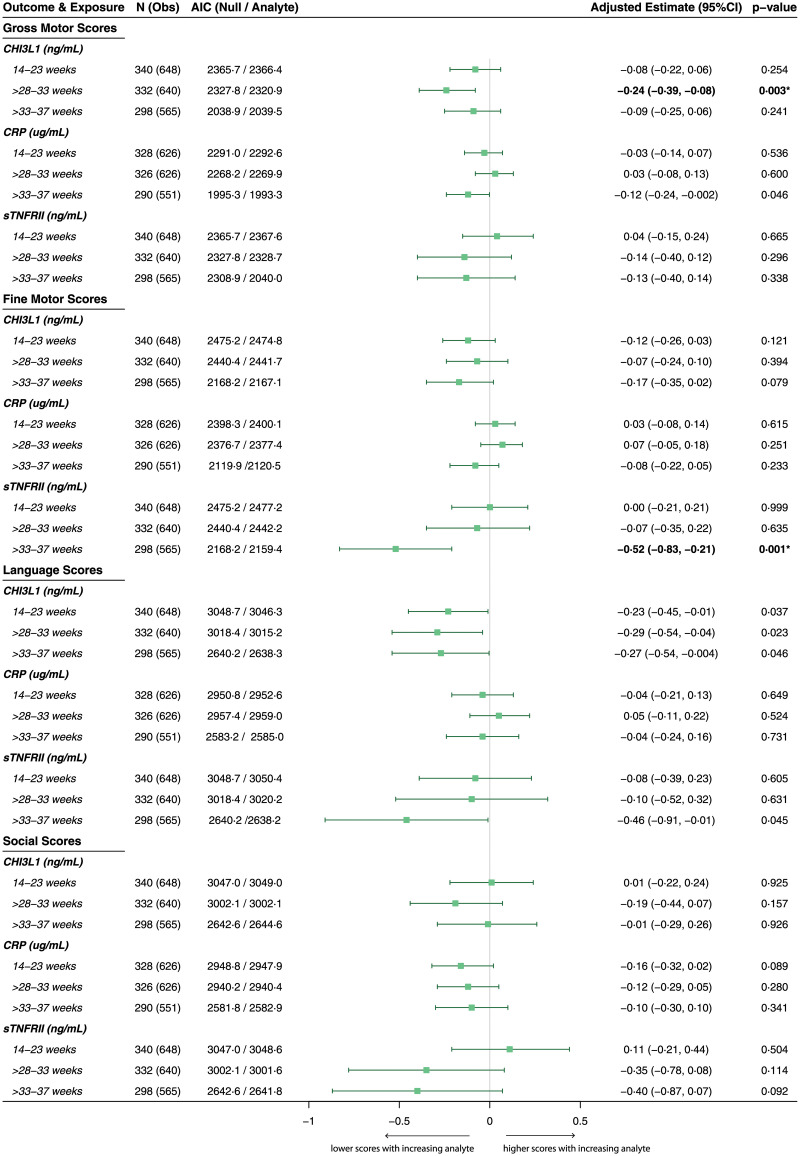
Fig 5. Longitudinal MDAT subdomain scores in children by maternal inflammatory mediator exposure.
Results of linear mixed-effects models for repeated neurocognitive score measures over time (12, 18, and 24 months) by maternal immune activation. Maternal immune activation defined by inflammatory analyte concentrations by gestational age (in weeks) at time of sample acquisition. N represents the total number of children who had both a neurocognitive score and a corresponding maternal analyte measurement at the respective gestational age, and Obs represents the number of observations (scores) included in each model. Possible range of MDAT subdomain scores (minimum/maximum scores in this cohort across all visits): gross motor, 12–27; fine motor, 11–29; language, 1–27; social, 11–33. AIC values (parameter of model fit), adjusted beta estimates (change in raw neurocognitive score for a 1-unit increase in analyte, holding other fixed effects constant) with 95% CIs, and likelihood ratio test results (p-values) are presented. All models adjusted for maternal age, maternal socioeconomic status, treatment arm, Family Care Indicator score, birth weight, corrected age of child at assessment, number of childhood malaria infections, and sex of child as fixed effects, and a by-participant intercept as a random effect. p-Value determined by likelihood ratio test comparing model with analyte to null model (without analyte). Analyte measurements were log-transformed. Uncorrected p-values are presented; two associations remained statistically significant after adjustment for multiple comparisons according to the Holm–Bonferroni method (4 analyte measurements × 3 analytes; n = 12 comparisons) (in bold and marked by an asterisk). AIC, Akaike information criterion; CHI3L1, chitinase-3-like protein 1; CRP, C-reactive protein; MDAT, Malawi Developmental Assessment Tool; sTNFRII, soluble tumor necrosis factor receptor II.