Fig. 2 |. A guide to using extracellular flux assays for glycolysis.
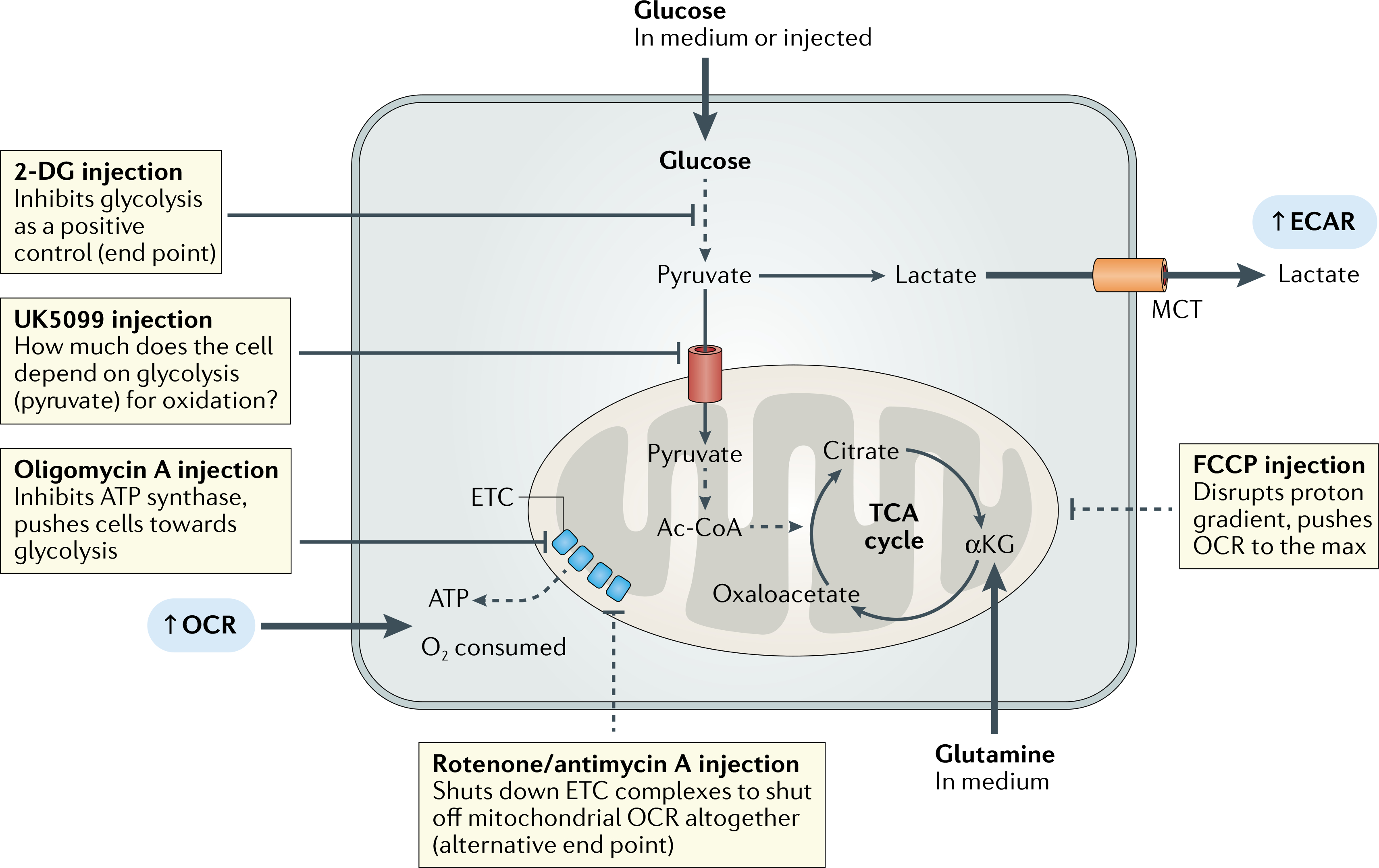
Glycolysis can be probed qualitatively or quantitatively and with respect to oxidative metabolism when paired with a mitochondrial stress test. If the cellular response to acute glucose availability is unknown, the extracellular acidification rate (ECAR) can be monitored after glucose is injected. Ultimately, a rise in ECAR due to lactate accumulation is dependent on lactate excretion via monocarboxylate transporters (MCTs). Glucose may be injected as a first step to monitor glucose consumption in real time, followed by an oligomycin A injection to force glycolytic function (glycolysis stress test). Next, 2-deoxy-d-giucose (2-DG) can be used as an assay end point to get a full picture of the glycolytic capacity of the cells. If the user is interested in the importance of glycolysis with respect to oxidative metabolism, UK5099 can be used to block pyruvate import through the mitochondrial pyruvate carrier (MPC), which prevents pyruvate from being converted into acetyl-CoA (Ac-CoA) and fuelling the tricarboxylic acid (TCA) cycle. Mitochondrial stress test injections in MPC-inhibited cells are used to compare rates of maximal mitochondrial respiration between UK5099-treated samples and untreated controls. αKG, α-ketoglutarate; ETC, electron transport chain; FCCP, carbonyl cyanide-4-trifluoromethoxyphenylhydrazone; OCR, oxygen consumption rate.
