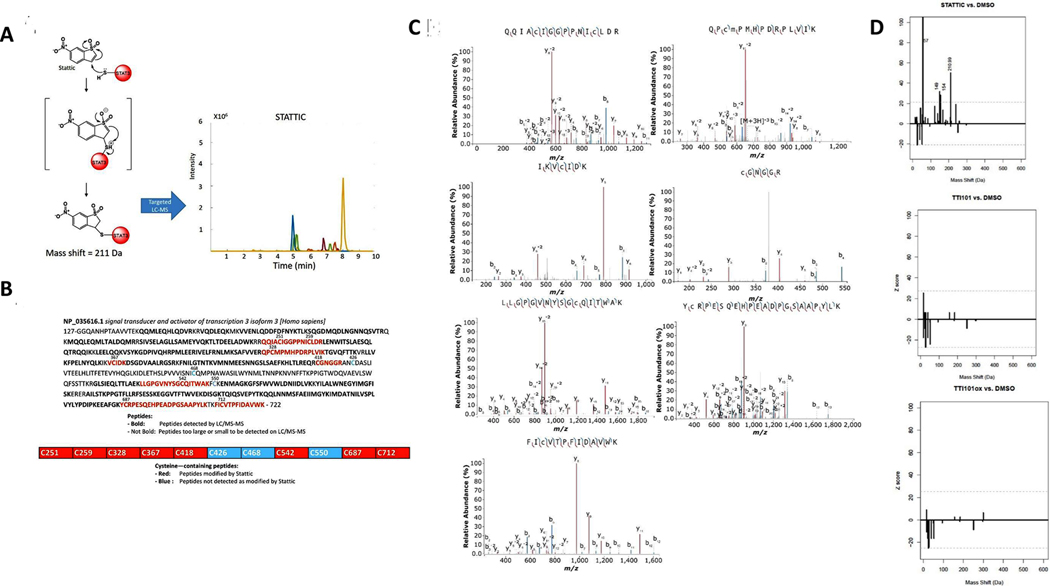
Fig. 5.
Alkylation of STAT3 by Stattic. Schematic depicting chemistry of possible alkylation of STAT3 by Stattic and results of LC-MS chromatograms of STAT3 peptides of alkylated peptides, as predicted from reaction chemistry. Results of LC-MS/MS demonstrating covalent modification of STAT3 by Stattic. Chromatograms show fragment ion analysis revealing alkylation of each cysteine-containing peptide, as indicated. Mass Spectra were annotated using IPSA [47]. Representative data of four independent experiments. Amino acid sequence of STAT3βtr indicating cysteine residues modified. Red residues indicates cysteine-containing tryptic fragments identified by LC-MS/MS, with some peptides containing more than one modified cysteine. Bolded residues are within tryptic fragments that can be identified by LC-MS/MS. Residues that are not bolded are within tryptic fragments that are either too large or too small to be detected. Z-score histograms comparing mass shifts of STAT3 peptides incubated with Stattic, TTI-101, or TTI-101ox vs. STAT3 incubated with DMSO. The dotted line indicates the cutoff for a significant Z-score. The peptide mass peak shifted by 211 Da represents the predicted addition of Stattic as a chemical adduct; no significant mass shifts were observed with TTI-101 or with TTI-101ox indicating that neither forms chemical adducts with STAT3.