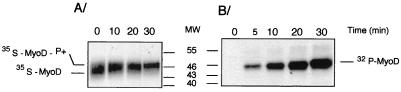
FIG. 7.
MyoD is a substrate of cyclin E-Cdk2-dependent phosphorylation. (A) In vitro-translated 35S-labeled MyoDwt was incubated with purified cyclin E-Cdk2 complexes for various periods of time. Phosphorylation shifts were visualized after SDS-PAGE. Shown is the autoradiogram of the SDS-PAGE analysis. (B) Bacterially produced MyoD protein was phosphorylated by purified cyclin E-Cdk2 complex for the time indicated. Shown is the autoradiogram for 32P phosphorylation reactions. Unphosphorylated MyoD protein migrates as 45-kDa band that progressively shifts to 47 kDa in the course of phosphorylation by cyclin E-Cdk2 kinase reaction. Positions of molecular weight markers (MW) are shown in thousands.