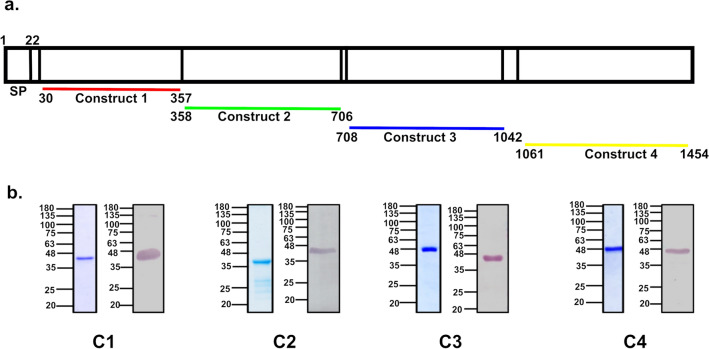
Figure 1.
Expression of PfMSA180 as four recombinant proteins in E. coli and generation of specific antibodies. PfMSA180 is synthesized as a ~ 180 kDa protein in the parasite with a predicted N-terminal signal peptide (SP). The four recombinant proteins were expressed from plasmid constructs that together span the entire length of the protein. The constructs were based on the pET24b vector for expression in E. coli and the proteins were purified by Immobilised Metal Affinity Chromatography (IMAC) and size exclusion chromatography. (a) Schematic representation of different constructs designed for PfMSA180. The protein possesses a signal peptide (SP; residues 1–22). (b) Purified PfMSA180 recombinant proteins: Construct 1 (C1) spans the N-terminal region of the protein (residues N30-T357) and has a predicted molecular mass of 39 kDa, Construct 2 (C2; residues N358-N706) has a predicted molecular mass of 41.5 kDa, Construct 3 (C3; residues N708-D1042) has a predicted molecular mass of 40 kDa, and Construct 4 (C4) spans the C-terminal region of the protein (residues N1061-N1454) and has a predicted molecular mass of 47 kDa. The purified proteins were separated by SDS-PAGE and stained with Coomassie blue, as well as transferred and their identity and molecular mass confirmed by immunoblotting with anti-6XHis antibody. Full length gels and immunoblots are presented in Supplementary Fig. 8. The lower bands observed on Coomassie blue-stained gels of C2, C3 and C4 were not specifically identified by anti-His antibody, suggesting slight degradation of the protein during refolding and dialysis.