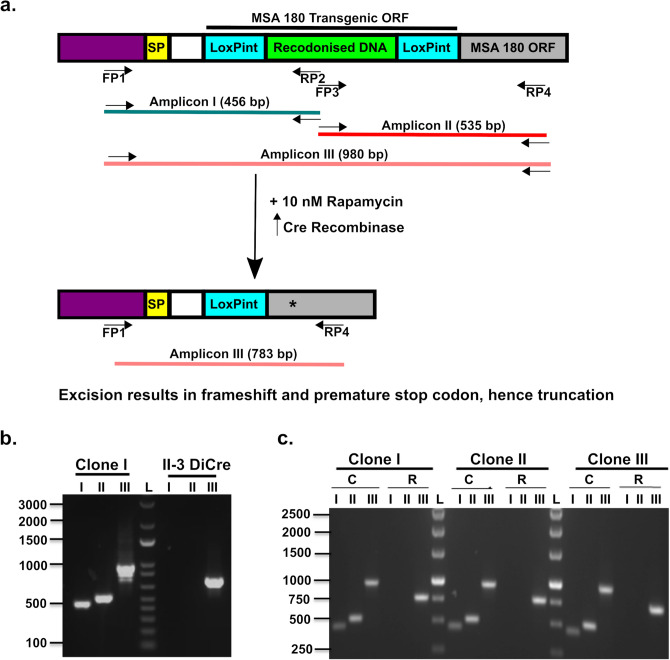
Figure 5.
Generation of parasite lines to perform inducible knockout of the pfmsa180 gene. (a) Schematic diagram of the expected amplicons with different primer pairs. The recondonised DNA between the two LoxPint sites was used to differentiate the transfected (PfMSA180) and wild type (II-3 DiCre) parasite lines. SP- Signal peptide, FP- Forward Primer, RP- Reverse primer, *premature stop codon after successful recombination, LoxPint- LoxP sites in sera2 intron. Predicted amplicon I (456 bp) was amplified with primer pairs 1/2, Amplicon II (535 bp) was amplified with primer pairs 3/4, Amplicon III was amplified with primer pairs 1/4 and the size depended on rapamycin treatment (without rapamycin/DMSO treated, 980 bp; after rapamycin treatment, 783 bp). The transgenic ORF is comprised of two LoxPint sites inserted at the 5’ end of the gene; the remaining msa180 ORF is depicted by grey box. Not to scale. (b) PCR to confirm transfection and insertion of repair sequence. Lane1- Amplicon I (Primers 1/2), Lane 2- Amplicon II (Primer 3/4), Lane 3- Amplicon III (Primers 1/4) from pfmsa180 inducible knockout clone (980 bp) and from wildtype II-3 DiCre parasites (774 bp). PCR results shown for one of three pfmsa180 inducible knockout parasite clones. PfMSA180 transgenic line – the presence of a band in Lane 1 and 2 of MSA180 confirms integration of the repair sequence containing two LoxPint sites into the parasite genome. Lane 3 confirms the presence of unmodified msa180 sequence in wildtype parasites. (c) PCR analysis of DNA excision after rapamycin treatment for all three inducible msa180 knockout clones. Early ring-stage parasites were treated with 10 nM rapamycin and the growth medium was changed after 24 h without adding further rapamycin. L- DNA Ladder, Lane1-Amplicon I (Primers 1/2), Lane 2-Amplicon II (Primer 3/4), Lane 3-Amplicon III (Primers 1/4). DMSO-treated PfMSA180 (control)—980 bp, Rapamycin-treated—783 bp. Absence of bands in lanes 1 and 2 following rapamycin treatment confirms successful gene excision. Full length picture of the agarose gel showing PCR product bands and DNA ladder can be found in Supplementary Fig. 10.