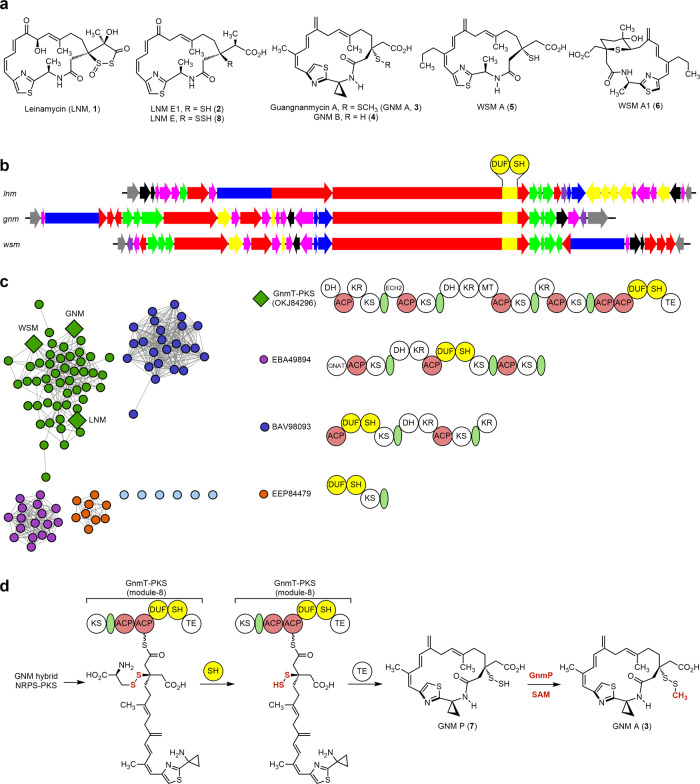
Fig. 1. GNM biosynthesis as a model for the LNM family of natural products featuring a thiocysteine lyase as a PKS domain that directly installs an -SSH group into the GNM hybrid peptide–polyketide scaffold.
a The structures of LNM (1), GNM A (3), and congeners LNM E1 (2), LNM E (8), GNM B (4), WSM A (5), and WSM A1 (6) (Supplementary Fig. 3). b The lnm, gnm, and wsm BGCs featuring DUF-SH didomain-containing type I PKSs. c Sequence similarity network (SSN) of 109 DUF-SH domain-containing PKS proteins revealing four major clusters, with representative PKS modular architectures shown for the four major clusters (see Supplementary Figs. 4 and 5 for a complete summary). The DUF-SH didomain-containing PKS proteins from the LNM-type BGCs are color-coded in green, with the corresponding PKSs from the LNM, GNM, and WSM BGCs depicted as diamonds. d The biosynthetic pathway of 3 highlighting (i) GnmT-SH domain-catalyzed installation of the -SSH group via an L-thiocysteine adduct at C-3 of the growing polyketide chain, (ii) hydropersulfide 7 as the nascent product of the GNM hybrid NRPS-PKS assembly line, and (iii) GnmP-catalyzed S-methylation of 7 to afford 3. ACP acyl carrier protein, DH dehydratase, DUF domain of the unknown function, ECH2 enoyl-CoA hydratase homolog 2, GNAT GCN5-related N-acetyltransferase, KR ketoreductase, KS ketosynthase, MT methyltransferase, SH thiocysteine lyase, TE thioesterase, and the green ovals denoting an acyltransferase docking domain.