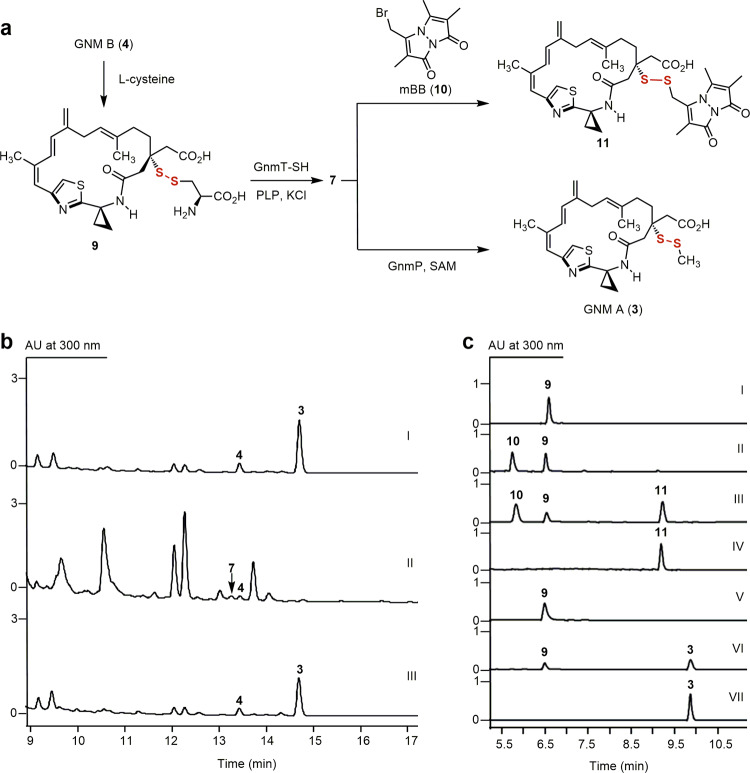
Fig. 2. In vivo and in vitro characterization of GnmP as a SAM-dependent hydropersulfide methyltransferase, establishing 7 as the penultimate intermediate for 3 biosynthesis.
a Chemoenzymatic synthesis of 7 from 4 and a GnmT-SH and GnmP-coupled assay of GnmP-catalyzed S-methylation of 7 to 3 using 9 as a surrogate substrate. b HPLC analysis of metabolite profiles: (I) S. sp. CB01883 (wild-type), (II) SB21007 (ΔgnmP), and (III) SB21008 (ΔgnmP/gnmP). c HPLC analysis of in vitro assays: (I) substrate 9, (II) 9 + GnmT-SH (boiled) + 10, (III) 9 + GnmT-SH + 10, (IV) product 11, (V) 9 + GnmT-SH (boiled) + GnmP (boiled) + SAM, (VI) 9 + GnmT-SH + GnmP + SAM, and (VII) product 3. Substrate and enzyme concentrations used: 9, 0.5 mM; SAM, 2 mM; GnmT-SH, 10 μM; and GnmP, 30 μM. Incubation time: 20 min. The concentration of 10 used to trap 7 in situ as 11: 0.5 mM. “AU” denotes absorbance units.