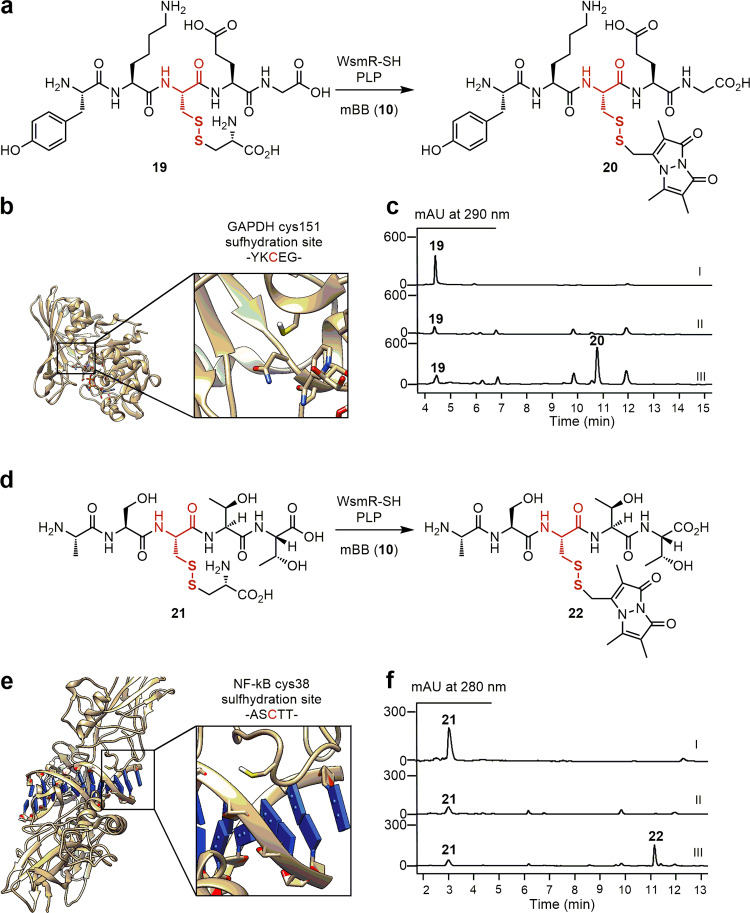
Fig. 5. Leveraging the SH domains as biocatalysts to install an -SSH group into varying peptide scaffolds.
WsmR-SH-catalyzed synthesis of a 20 and d 22 from the l-thiocysteine-pentapeptide adducts 19 and 21 as substrate mimics for GAPDH and NF-kB, respectively. The crystal structures of b GAPDH (PDB: 1ZNQ) and e NF-kB (PDB: 1LE9) highlighting the Cys151 and Cys38 residues that undergo sulfhydration, respectively. HPLC analysis of in vitro assays of c (I) substrate 19, (II) 19 + WsmR-SH (boiled) + 10, and (III) 19 + WmR-SH + 10 and f, (I) substrate 21, (II) 21 + WsmR-SH (boiled) + 10, and (III) 21 + WmR-SH + 10. Substrate and enzyme concentrations used: 19 or 21, 2 mM; and WsmR-SH, 30 μM. Incubation time: 30 min. The concentration of 10 used to trap the resultant hydropersulfide product in situ as 20 or 22, respectively: 2 mM.