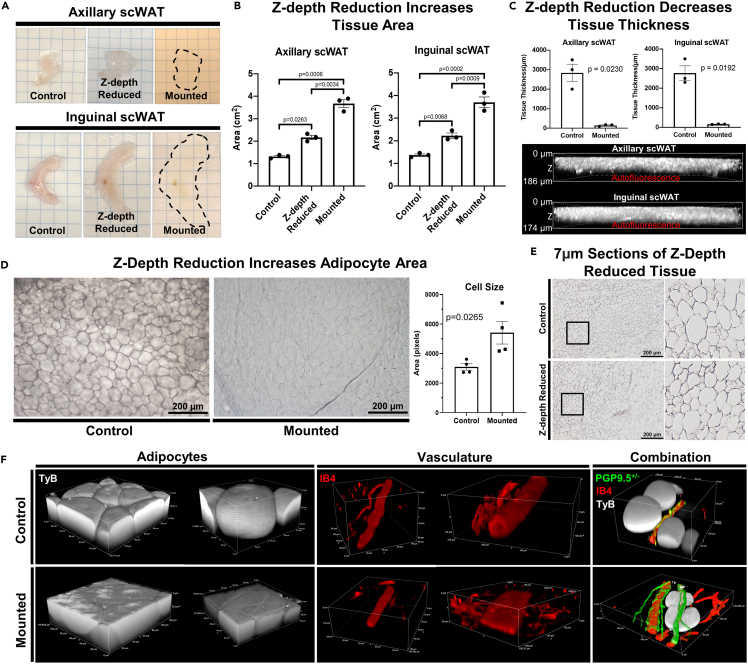
Figure 2.
Z-depth reduction (ZDR) and tissue morphology
(A) Axillary subcutaneous white adipose tissue (axi-scWAT) and inguinal (ing-scWAT) depots were Z-depth reduced and mounted on slides.
(B) Tissue area was measured before ZDR (control), after ZDR, and after mounting. N = 3, paired two-tailed Student’s t-test, alpha level 0.05, error bars are SEMs.
(C) Tissue thickness in the z-axis was measured at the thickest point before and after ZDR and mounting. Mounted tissues were measured by 3D projecting tissue autofluorescence captured on a Nikon A1R with a 20X objective. N = 3, paired two-tailed Student’s t-test, alpha level 0.05, error bars are SEMs.
(D) Ing-scWAT was either whole mount processed (Z-depth reduced) or left unaltered (control) and placed on a slide and imaged with transmitted light. Four representative 10X micrographs were captured for each tissue and the area for 30–120 cells was measured per micrograph and analyzed using a two-tailed Student’s t-test, Alpha level 0.05, error bars are SEMs.
(F) Hematoxylin staining of 7 μm ing-scWAT sections that either received ZDR before paraffin embedding or did not (control). Both ing-scWAT depots were excised from a PGP9.5+/− (green) direct reporter mouse (C57BL/6-Tg(Uchl1-EGFP)G1Phoz/J) and co-stained with IB4 (vasculature, red) and DAPI (nuclei, blue). One depot was Z-depth reduced and mounted whereas the other was left uncompressed to demonstrate morphological changes introduced by compressing the tissue; demonstrated through 3D reconstructions of similar structures.
Other details: Transmitted light micrographs were captured on a Nikon E400 upright microscope (D and E). Confocal micrographs captured on Nikon A1R (C and F). Scale bars are 186 μm and 174 μm (C), 200 μm (D and F) and 50 μm (E).