Abstract
Introduction:
Symptoms are the most common indication for ablation in children with atrioventricular nodal reentrant tachycardia (AVNRT). After the procedure, patients may continue to report palpitations. The objective of this study was to quantify the risk and duration of palpitations after pediatric slow pathway modification as well as demographic and technical associations.
Methods:
This was a retrospective review of consecutive patients at a pediatric center who underwent slow pathway modification for AVNRT from 2012–2018. Patients with a prior ablation attempt or congenital heart disease were excluded.
Results:
Palpitations were documented in 35% of patients after ablation. Neither post-ablation echo beats nor other evidence of residual dual AV nodal physiology were associated with a higher risk of post-ablation palpitations. Of the 35 patients with post-ablation palpitations, the median time to resolution of palpitations was 48 months. Acute procedural success was achieved in all 100 cases. There were two recurrences of AVNRT during long-term follow-up and one instance of ectopic atrial tachycardia (3% SVT recurrence).
Conclusion:
Palpitations after AVNRT ablation occurred in approximately one-third of cases, despite a low recurrence of true arrhythmia. Prior to ablation, patients and families should be counseled that post-ablation palpitations are common and AVNRT recurrence is rare.
Keywords: AVNRT, SVT, palpitations, ablation, cryoablation
Introduction
Palpitations are the most common symptom associated with atrioventricular nodal reentrant tachycardia (AVNRT). AVNRT is a paroxysmal arrhythmia that occurs when dual AV nodal physiology supports a reciprocating tachycardia. It is a common form of supraventricular tachycardia (SVT) in adolescents and young adults [1]. Symptoms may impact family life, frequency of medical care, and school attendance. Although AVNRT is not typically life-threatening, children with SVT have decreased total quality of life scores [2]. Many patients with symptomatic AVNRT undergo an electrophysiology (EP) study with ablation and receive slow pathway modification with either cryoablation or radiofrequency (RF) energy. Elimination of the slow pathway eliminates the substrate for SVT. Ideally, palpitations should resolve.
Despite the high rate of procedural success in the modern era [3–18], some patients report post-ablation palpitations [19–21]. The objective of this study was to determine the risk of palpitations after AVNRT ablation in children. We also studied technical and demographic associations with post-ablation palpitations, time to symptom resolution, and AVNRT recurrence.
Methods
We performed a retrospective review of 100 consecutive patients who underwent ablation for AVNRT from January 2012 through August 2018 at a single tertiary pediatric center. Three authors (TC, LD, GW) reviewed study reports and tracings to ensure there were either diagnostic intracardiac AVNRT tracings or evidence of dual AV nodal physiology with prior outpatient documentation of narrow complex tachycardia. We excluded patients with a prior ablation attempt and patients with congenital heart disease other than a patent foramen ovale or physiologic valve regurgitation.
Holter and event monitors were tabulated starting the day after post-ablation discharge. We did not record patient-reported palpitations that occurred within the first 30 days after ablation, unless a monitor had been sent. We chose to institute this 30-day delay in our retrospective review because we counsel all patients to expect isolated palpitations in the first month after slow pathway modification. Therefore, we do not expect our medical record to accurately reflect the percentage of patients with palpitations during that period. Following the 30-day period, we reviewed all clinic visits and phone encounters for patient-reported palpitations.
We defined palpitations as a written record in the chart of “palpitations” or other words that suggest sustained arrhythmia symptoms (e.g. “fluttering”, “fast heartbeat”). However, we did not include words that suggest instantaneous complaints (e.g. “hard beat”, “skipped beat”) or common complaints that are unreliably associated with arrhythmia (e.g. “chest pain”). We were unable to record the frequency of palpitations or additional details regarding the character of palpitations due to inconsistent documentation in the medical record.
We reviewed technical aspects of the ablation including type of AVNRT, type of ablation energy (RF or cryoablation), number of ablation lesions, duration of ablation lesions, and ablation catheter tip length. Similar to prior publications, we used a linear cryoablation technique that placed a series of lesions from the annulus of tricuspid valve through the posterior one-third of Koch’s triangle [3,7,10]. We performed RF ablation as previously described [4]. All intracardiac tracings were reviewed for evidence of dual AV nodal physiology after ablation. We tabulated any evidence of remaining dual AV nodal physiology: (1) single echo beats; (2) atrial-to-His (AH) jump, defined as an increase in the AH interval greater than 50 ms per 10 ms decrement during atrial extra-stimulus testing; (3) ventricular-to-atrial (VA) jump, using the same millisecond criteria; (4) sustained slow pathway conduction (SSPC) where the AV interval is longer than the programmed A-A interval during atrial incremental pacing due to a prolonged AH interval. Residual dual AV nodal physiology was defined as the presence of one or more of these findings on testing after the last ablation lesion.
Patients were scheduled for clinic visits at 3 and 12 months post-ablation. Patients who were asymptomatic with no complications 12 months after ablation were typically discharged from scheduled follow-up. Ambulatory monitors were 24-hour full-disclosure Holter monitors or event monitors (Mednet Healthcare Technologies, Ewing, NJ). Event monitors were either looping (electrodes affixed to the chest 24 hours/day) or non-looping monitors (placed on the chest at the patient’s discretion). Event monitors were typically prescribed for 30 days, and the type of event monitor was chosen per the treating physician’s discretion.
Statistical Methods
Demographic characteristics were summarized by mean and standard deviation or median and interquartile range, as appropriate. A Wilcoxon rank-sum test was used for nonparametric comparison of follow-up duration between those with palpitations after ablation and those without palpitations after ablation. An unadjusted logistic regression model followed by multivariable analysis was used to test associations of demographic characteristics and technical aspects of the procedure with post-ablation palpitations.
For those patients with palpitations after ablation, time to resolution of palpitations was analyzed with a nonparametric survival analysis for interval-censored data. Resolution of palpitations was defined as the first encounter with no palpitations, after which there were no subsequent encounters with palpitations. Patients who reported palpitations at their last follow-up visit were treated as censored at the date of the last follow-up, including in cases where patients transferred care or were lost to follow-up. Due to interval censoring, the median time-to-resolution was estimated by imputing survival probabilities within non-overlapping intervals [22,23].
Results
In our retrospective review (mean age 14.2 years ± 3.7, range 5–24), 35 patients (35%) reported palpitations after ablation. The median time to resolution of palpitations was 47.7 months (Figure 1). Among the 35 patients with palpitations, 15 became asymptomatic prior to censoring. The remaining 20 patients still reported palpitations in their most recent encounter and were therefore right-censored in the analysis. We did not observe a statistically significant increase in odds of post-ablation palpitations based on sex, age, or weight (Table 1). The chief complaint prior to ablation was symptomatic SVT in 99/100 cases with one case of asymptomatic SVT on ambulatory monitoring. Median duration of cardiology follow-up was 364 days (IQR 99, 557) but differed substantially between patients without palpitations (median 163 days, IQR 83, 409) and with palpitations (median 666 days, IQR 391, 958), p<0.0001).
Figure 1.
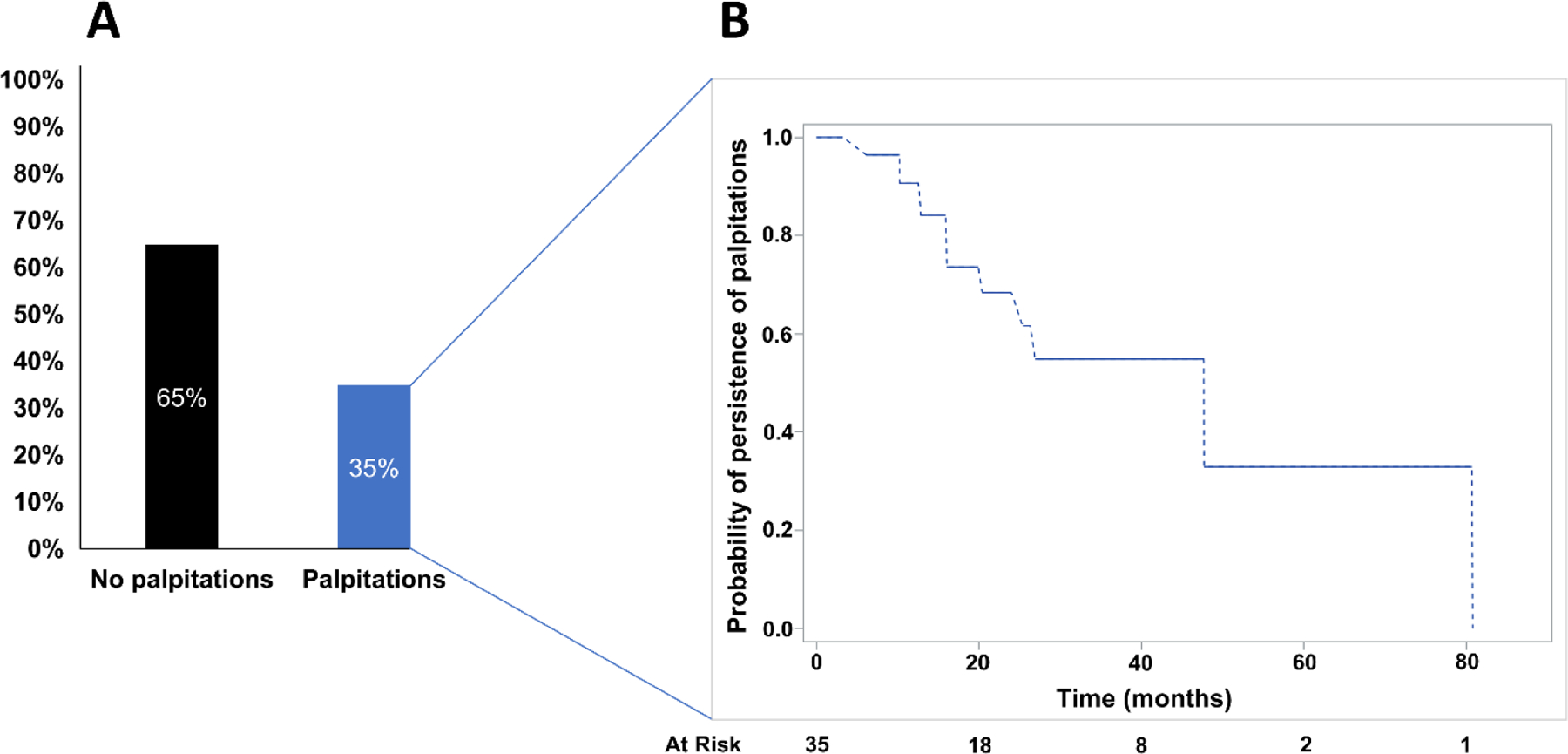
Nested diagram of palpitations after AVNRT ablation and time to resolution of symptoms. All patients in the study are represented in Figure 1A, demonstrating the fraction without palpitations (black) and with palpitations (blue) after ablation. In Figure 1B, the subgroup with palpitations is tabulated using an interval-censored Kaplan-Meier survival function, demonstrating time to resolution of palpitations. At risk numbers are provided assuming right censoring.
Table 1.
Patient Demographics.
| All subjects (n = 100) |
Palpitations after ablation (n = 35) |
No palpitations after ablation (n = 65) |
P-value† | |
|---|---|---|---|---|
| Female (%) | 60 (60) | 24 (69) | 36 (55) | 0.20 |
| Age, years ± SD | 14.2 ± 3.7 | 14.3 ± 3.5 | 14.2 ± 3.9 | 0.83 |
| Weight, kg (IQR) | 57.5 (46.1, 68.6) | 57.0 (40.8, 69.2) | 57.8 (47.6, 68.4) | 0.85 |
P-value from logistic regression model
SD = standard deviation; IQR = interquartile range
All 100 ablation patients had acute procedural success (87 typical AVNRT, 13 atypical AVNRT). Neither inducible SVT nor non-sustained arrhythmia were present during post-ablation pacing in any patients after the final ablation. On post-ablation testing, 54/100 patients had evidence of dual AV nodal physiology. Isolated AH jump was present in 24/100 cases; VA jump was present in 9/100 cases; echo beats were present in 43/100 cases; and SSPC was present in 2/100 cases. No patients were discharged from the procedure with more than two findings of residual dual AV nodal physiology. There was no association between type of AVNRT or evidence of residual dual AV nodal physiology and odds of palpitations (Table 2).
Table 2.
Technical aspects of ablation.
| All subjects (n = 100) |
Palpitations after ablation (n = 35) |
No palpitations after ablation (n = 65) |
P-value† | ||
|---|---|---|---|---|---|
| Type of AVNRT | |||||
| Atypical | 13 | 5 | 8 | ||
| Cryoablation catheter tip length | |||||
| 8mm | 55 | 14 | 41 | ||
| Total duration of cryoablation in seconds (IQR) | 3463 (2407, 4811) | 3377 (2411, 4809) | 3532 (2400, 5048) | 0.53 | |
| Number of cryoablation lesions (IQR) | 15 (11, 22) | 15 (12, 21) | 15 (11, 23) | 0.55 | |
| Echo beats | |||||
| Yes | 43 | 14 | 29 | ||
| Residual dual AV nodal physiology | |||||
| Yes | 54 | 19 | 35 | ||
P-value from logistic regression model, except for cryoablation catheter tip length, which was subjected to multivariate analysis to control for potential confounding.
IQR = interquartile range
Three patients had documented SVT after ablation (3% of the cohort). All three patients had post-ablation palpitations. Of these, two had recurrence of AVNRT (2% AVNRT recurrence risk), diagnosed by ambulatory monitor within three months of ablation. One of the patients with AVNRT recurrence subsequently underwent a successful repeat slow pathway modification with radiofrequency energy. After her second ablation, she continued to report palpitations but had no further recurrence of SVT. The other patient with AVNRT recurrence had documented AVNRT 4 days after ablation and was treated with atenolol. The third patient had documented SVT 26 days after the ablation, and a follow-up EP study diagnosed ectopic atrial tachycardia (EAT) near the triangle of Koch. In reviewing the first study, it was likely that the patient had EAT in both procedures. This case was included on an intention-to-treat basis because the electrophysiologist at the first study diagnosed AVNRT and performed slow pathway modification with an apparently successful endpoint at the end of the first case.
Both AVNRT recurrences occurred in the cryoablation group, but cryoablation was the only modality in 93% of cases (2.2% AVNRT recurrence risk among patients who had cryoablation-only). Three patients had RF ablation alone; four patients had both cryoablation and RF ablation; and seven patients had RF ablation of an accessory pathway in addition to slow pathway cryoablation. There were no associations between technical aspects of the procedure and odds of post-ablation palpitations (Table 2). Palpitations were present in 31/93 patients with cryoablation only, 2/4 patients with cryoablation + RF, and 2/3 patients with RF only.
Two patients carried a diagnosis of postural orthostatic tachycardia syndrome (POTS) prior to ablation. Both patients had palpitations after ablation. In addition, one patient with post-ablation palpitations received a new diagnosis of POTS after ablation. None of the patients with POTS had SVT recurrence.
In 10 of the 35 patients with palpitations after ablation, reassurance from the electrophysiologist was sufficient without additional monitoring (29%). One or more ambulatory monitors were prescribed in 25 of the 35 patients with palpitations (71%). Holter monitors and event monitors were used with similar frequency (37 event monitors and 43 Holter monitors). Multiple monitors were prescribed in 22/25 patients, and 8/25 patients received four or more monitors (Figure 2). Of the 25 patients who received monitors, rhythm-symptom correlation was recorded in 24 patients. SVT recurrence was documented in three patients, as reported above. Monitoring revealed symptoms that correlated with isolated atrial or ventricular ectopy in four patients. In the remaining 17 patients, all symptoms correlated with sinus rhythm on monitors. Thus, there were 11 patients who had post-ablation palpitations and no rhythm-symptom correlation, either because no symptoms were captured on an ambulatory monitor or no monitor was prescribed. A sensitivity analysis excluding the 11 patients without rhythm-symptom correlation and the 3 patients with SVT recurrence yields only a slightly lower incidence of post-ablation palpitations (24%). All patients were included in subsequent analyses to avoid selection bias.
Figure 2.
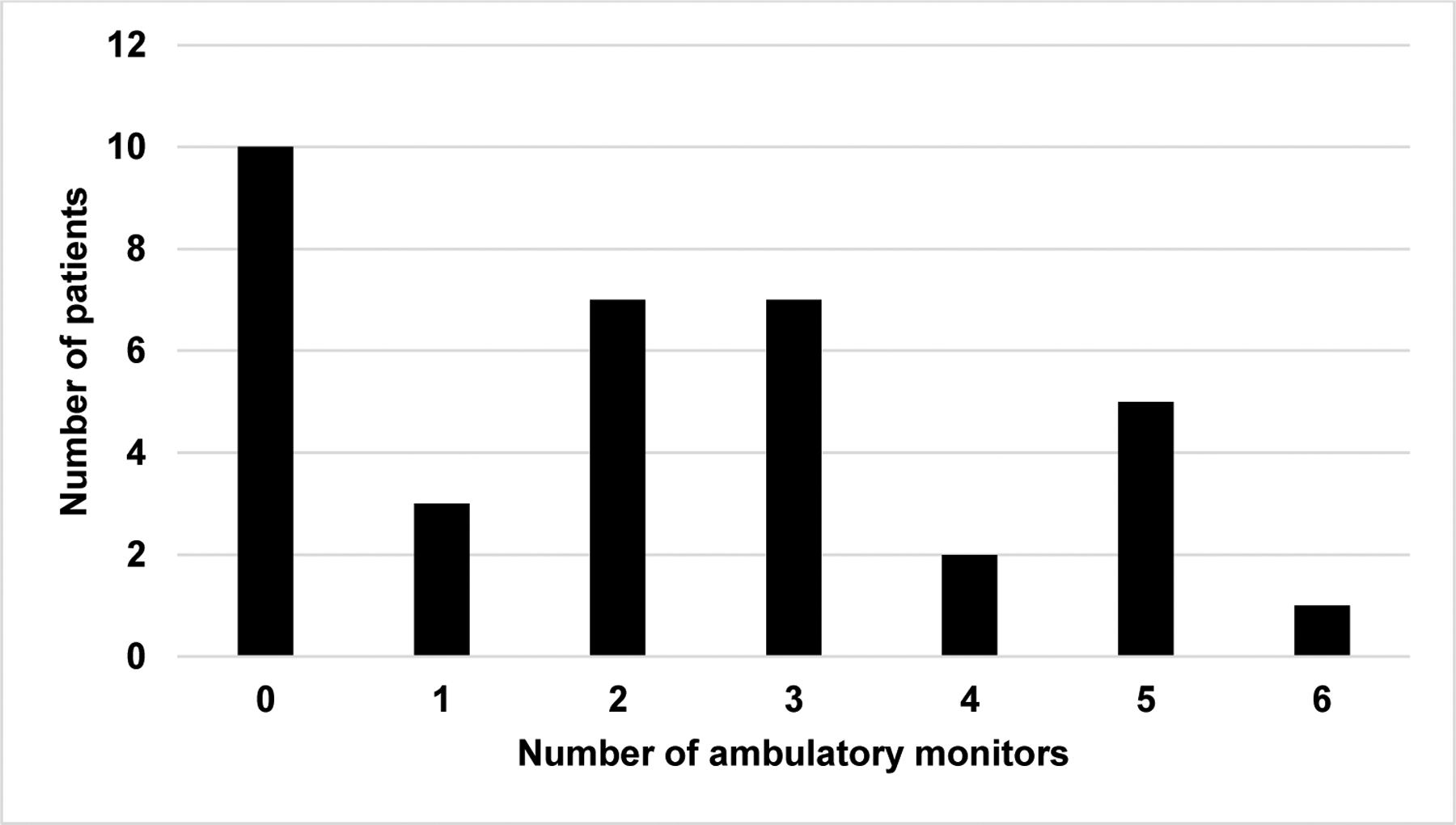
Quantification of ambulatory arrhythmia monitors among patients who continued to experience palpitations after ablation. The number of ambulatory monitors (77 total) includes 30-day event monitors and 24-hour Holter monitors.
Three patients without palpitations received Holter monitors. One had frequent asymptomatic SVT prior to ablation, and the electrophysiologist wanted to ensure the patient had no further asymptomatic SVT. One had Wenckebach on inpatient telemetry after ablation. One had frequent atrial ectopy during the EP study. None of these patients had documented SVT after ablation.
Discussion
In this study, 35% of children and young adults with structurally normal hearts who underwent a first ablation procedure for AVNRT continued to report palpitations 30 days or more after the procedure. There is scant literature available on this topic. One study of 63 subjects demonstrated post-ablation palpitations risk of 30% for RF and 43% for cryoablation in AVNRT [19]. Another older study suggested a “major” palpitations risk of 36% after RF ablation for SVT [20]. We counsel patients that palpitations after AVNRT are common, but recurrence is rare.
In our study, most patients with palpitations after ablation continued to report them for months to years. However, we do not counsel families that it will necessarily take years for palpitations to go away. We believe that the duration of palpitations is affected by the retrospective nature of our study; patients typically stop calling the doctor when their palpitations resolve. Therefore, our retrospective results are skewed to have more encounters and a longer follow-up duration in patients with more severe palpitations.
It is not clear why palpitations persist even without recurrence of AVNRT. One plausible theory is that arrhythmia patients have a heightened awareness of their heart rhythm. Thus, benign irregularities such as premature atrial contractions, premature ventricular contractions, or paroxysmal sinus tachycardia may be perceived as palpitations [20]. This is consistent with our low recurrence and high number of benign ambulatory monitors during follow-up, in which symptoms correlated with either sinus rhythm or isolated ectopy. For now, we can counsel families what to expect, but we cannot provide a universal explanation as to why palpitations may persist. Regardless of the etiology, the duration of symptoms in some patients makes it important to explain to families before ablation that not all post-ablation palpitations are SVT.
There were three patients in this study with a diagnosis of POTS in addition to AVNRT, two of which had this diagnosis prior to ablation. All three of these patients had persistence of palpitations after ablation without SVT recurrence. This is not surprising since palpitations are a common symptom of POTS [24]. Although the sample size of patients with POTS was too small for sub-group analysis, our data support the common practice of warning patients with POTS that palpitations may persist after AVNRT ablation.
Neither demographics nor technical aspects of the procedure influenced the risk of post-ablation palpitations. We did not identify any associations between age, sex, or weight and odds of post-ablation palpitations. None of the technical aspects of ablation were independently associated with the odds of palpitations after ablation, including duration of cryoablation, number of cryoablation lesions, and cryoablation catheter tip length. We found no association between type of AVNRT or residual dual AV nodal physiology and post-ablation palpitations.
Multiple ambulatory monitors were used for most of the patients with post-ablation palpitations. In our center, we plan to decrease the use of ambulatory monitors after AVNRT ablation, knowing that post-ablation palpitations are common and usually do not represent true arrhythmia. After a negative monitor with symptoms that correlate to sinus rhythm, cardiologists should prescribe additional monitors judiciously. We know from prior studies that late recurrences of AVNRT do occur [13], so a detailed history with attention to changes in quality, severity, and frequency of palpitations can be helpful in deciding to prescribe ambulatory monitors.
There has been an ongoing debate about RF energy versus cryoablation energy for AVNRT in a pediatric population. The two major factors in the current debate have been AVNRT recurrence and risk of complete heart block. Studies have shown a higher risk of SVT recurrence in patients who underwent cryoablation compared to RF ablation. In two meta-analyses, AVNRT recurrence for cryoablation ranged from 9.1–9.7% and for RF ablation ranged from 3.5–3.8% [25,26]. Therefore, the traditional trade-off has been between higher efficacy with a small risk of permanent heart block versus lower efficacy with no risk of heart block. Our AVNRT recurrence of 2.2% after cryoablation compares favorably with reported AVNRT recurrences after RF ablation and agrees with other recent pediatric studies with similarly low recurrence after AVNRT cryoablation (Table 3). As pediatric electrophysiologists have gained experience with cryoablation, recurrence risk seems to be decreasing, and our AVNRT recurrence risk is consistent with this overall trend.
Table 3.
Published risk of AVNRT recurrence in pediatric cohorts.
| First Author | Year | N | % SVT recurrence | Mean# follow-up duration, months | Mean# age, years | Ablation energy |
|---|---|---|---|---|---|---|
| Siebels18 | 2018 | 379 | 14 | 55 ± 40 | 13 | RF |
| Karacan17 | 2018 | 275 | 4 | 26 ± 14 | 11 | Cryo |
| Balli16 | 2018 | 109 | 1 | 13 ± 6 | 10 | Cryo |
| Tuzcu15 | 2017 | 125 | 10 (6-mm tip) 8 (8-mm tip) |
15 ± 8 | 14 | Cryo |
| Reddy14 | 2017 | 117 | 11 (control) 0 (voltage mapping) |
22 ± 17 (control) 15 ± 7 (voltage mapping) |
14 | Cryo |
| Backhoff13 | 2016 | 241 | 4–11, depending on duration of follow-up | 12–96 | 13 (median) | Cryo/RF |
| Reddy12 | 2015 | 239 | 1 | 48 ± 24 | 8 (young cohort) 15 (old cohort) | Cryo/RF |
| Kubus11 | 2014 | 205 | 9–16, depending on era | 14 (IQR 6–22) | 15 (median) | RF |
| Drago10 | 2014 | 202 | 11 | 18–25, depending on subject age | 12 | Cryo |
| Qureshi9 | 2013 | 51 | 2 | 31 ± 10 | 14 | Cryo |
| Das8 | 2012 | 246 | 7 | 24 ± 2 | 13 | Cryo |
| Silver7 | 2010 | 70 | 3 | 12 ± 3 | 15 | Cryo |
| Drago6 | 2010 | 74 | 7 | 30 (range 2–74) | 11 | Cryo |
| LaPage5 | 2010 | 61 | 7 | 36 ± 12 | 13 | Cryo |
| Fishberger4 | 2010 | 65 | 0 | 33 ± 17 | 12 | RF |
| Czosek3 | 2010 | 58 | 2 | 12 | 16 (median) | Cryo |
Cryo = cryoablation; RF = radiofrequency; ± denotes the reported standard deviation.
All data were drawn from the primary publication and rounded to the nearest whole number. Where median data were reported by the authors, data are reported here with interquartile range (IQR) or range instead of standard deviation.
Study Limitations
This is a single center retrospective study, with the inherent limitations of chart review, including limited information on causes of right-censoring. Additionally, the interval between telephone calls and clinic visits varied between patients. Accordingly, our time-to-symptom-resolution data is affected by the variability in these intervals. Given the retrospective nature of the study, we were unable to compare character or frequency of palpitations before and after ablation. While 99/100 patients had symptoms that correlated to SVT prior to ablation, not all pre-ablation symptoms were necessarily SVT. Therefore, pre- and post-ablation symptom comparison was not attempted.
Conclusion
Approximately one-third of patients report palpitations after ablation for AVNRT despite low risk of SVT recurrence (3%). Prior to ablation, patients and families should be counseled on the risk of post-ablation palpitations and the low risk of true recurrence.
Funding:
This work was supported by the National Institutes of Health, National Heart, Lung and Blood Institute, grant number K23HL130554; REDCap access was provided by Northwestern University Clinical and Translational Sciences Institute, funded in part by NIH UL1TR001422.
Footnotes
Disclosures: The authors have no conflicts of interest to report.
Ethics approval: This research study was conducted retrospectively from data obtained for clinical purposes. We consulted with the IRB of Ann & Robert H. Lurie Children’s Hospital of Chicago who determined that our study did not need ethical approval.
References
- 1.Porter MJ, Morton JB, Denman R, Lin AC, Tierney S, Santucci PA, Cai JJ, Madsen N, Wilber DJ. Influence of age and gender on the mechanism of supraventricular tachycardia. Heart Rhythm. 2004;1:393–6. [DOI] [PubMed] [Google Scholar]
- 2.Czosek RJ, Cassedy AE, Wray J, Wernovsky G, Newburger JW, Mussatto KA, Mahony L, Tanel RE, Cohen MI, Franklin RC, Brown KL, Rosenthal D, Drotar D, Marino BS. Quality of life in pediatric patients affected by electrophysiologic disease. Heart Rhythm. 2015;12:899–908. [DOI] [PubMed] [Google Scholar]
- 3.Czosek RJ, Anderson J, Marino BS, Connor C, Knilans TK. Linear lesion cryoablation for the treatment of atrioventricular nodal re-entry tachycardia in pediatrics and young adults. Pacing and Clinical Electrophysiology. 2010;33:1304–11. [DOI] [PubMed] [Google Scholar]
- 4.Fishberger SB, Whalen R, Zahn EM, Welch EM, Rossi AF. Radiofrequency ablation of pediatric AV nodal reentrant tachycardia during the ice age: a single center experience in the cryoablation era. Pacing and Clinical Electrophysiology. 2010;33:6–10. [DOI] [PubMed] [Google Scholar]
- 5.LaPage MJ, Saul JP, Reed JH. Long-term outcomes for cryoablation of pediatric patients with atrioventricular nodal reentrant tachycardia. The American Journal of Cardiology. 2010;105:1118–21. [DOI] [PubMed] [Google Scholar]
- 6.Drago F, Russo MS, Silvetti MS, De Santis A, Iodice F, Naso Onofrio MT. Cryoablation of typical atrioventricular nodal reentrant tachycardia in children: six years’ experience and follow-up in a single center. Pacing and Clinical Electrophysiology. 2010;33:475–81. [DOI] [PubMed] [Google Scholar]
- 7.Silver ES, Silva JN, Ceresnak SR, Chiesa NA, Rhee EK, Dubin AM, Avasarala K, Van Hare GF, Collins KK. Cryoablation with an 8-mm tip catheter for pediatric atrioventricular nodal reentrant tachycardia is safe and efficacious with a low incidence of recurrence. Pacing and Clinical Electrophysiology. 2010;33:681–6. [DOI] [PubMed] [Google Scholar]
- 8.Das S, Law IH, Von Bergen NH, Bradley DJ, Dick M 2nd, Etheridge SP, Saarel EV, Frias PA, Strieper MJ, Fischbach PS. Cryoablation therapy for atrioventricular nodal reentrant tachycardia in children: a multicenter experience of efficacy. Pediatric Cardiology. 2012;33:1147–53. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi MY, Ratnasamy C, Sokoloski M, Young ML. Low recurrence rate in treating atrioventricular nodal reentrant tachycardia with triple freeze-thaw cycles. Pacing and Clinical Electrophysiology. 2013;36:279–85. [DOI] [PubMed] [Google Scholar]
- 10.Drago F, Placidi S, Righi D, DI Mambro C, Russo MS, Silvetti MS, Palmieri R, Prosperi M. Cryoablation of AVNRT in children and adolescents: early intervention leads to a better outcome. Journal of Cardiovascular Electrophysiology. 2014;25:398–403. [DOI] [PubMed] [Google Scholar]
- 11.Kubuš P, Vít P, Gebauer RA, Zaoral L, Peichl P, Fiala M, Janoušek J. Long-term results of paediatric radiofrequency catheter ablation: a population-based study. Europace. 2014;16:1808–13. [DOI] [PubMed] [Google Scholar]
- 12.Reddy CD, Silka MJ, Bar-Cohen Y. A Comparison of AV nodal reentrant tachycardia in young children and adolescents: electrophysiology, ablation, and outcomes. Pacing and Clinical Electrophysiology. 2015;38:1325–32. [DOI] [PubMed] [Google Scholar]
- 13.Backhoff D, Klehs S, Müller MJ, Schneider HE, Kriebel T, Paul T, Krause U. Long-term follow-up after catheter ablation of atrioventricular nodal reentrant tachycardia in children. Circulation Arrhythmia and Electrophysiology. 2016;9. [DOI] [PubMed] [Google Scholar]
- 14.Reddy CD, Ceresnak SR, Motonaga KS, Avasarala K, Feller C, Trela A, Hanisch D, Dubin AM. Bridge to success: a better method of cryoablation for atrioventricular nodal reentrant tachycardia in children. Heart Rhythm. 2017;14:1649–54. [DOI] [PubMed] [Google Scholar]
- 15.Tuzcu V, Gul EE, Karacan M, Kamali H, Celik N, Akdeniz C. Comparison of 6-mm versus 8-mm-tip cryoablation catheter for the treatment of atrioventricular nodal reentrant tachycardia in children: a prospective study. Pediatric Cardiology. 2017;38:1220–5. [DOI] [PubMed] [Google Scholar]
- 16.Balli S, Kucuk M, Orhan Bulut M, Kemal Yucel I, Celebi A. Transcatheter cryoablation procedures without fluoroscopy in pediatric patients with atrioventricular nodal reentrant tachycardia: a single-center experience. Acta Cardiologica Sinica. 2018;34:337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karacan M, Celik N, Akdeniz C, Tuzcu V. Long-term outcomes following cryoablation of atrioventricular nodal reentrant tachycardia in children. Pacing and Clinical Electrophysiology. 2018;41:255–60. [DOI] [PubMed] [Google Scholar]
- 18.Siebels H, Sohns C, Nurnberg JH, Siebels J, Langes K, Hebe J. Value of an old school approach: safety and long-term success of radiofrequency current catheter ablation of atrioventricular nodal reentrant tachycardia in children and young adolescents. Journal of Interventional Cardiac Electrophysiology. 2018;53:267–77. [DOI] [PubMed] [Google Scholar]
- 19.Kimman GJ, Theuns DA, Janse PA, Rivero-Ayerza M, Scholten MF, Szili-Torok T, Jordaens LJ. One-year follow-up in a prospective, randomized study comparing radiofrequency and cryoablation of arrhythmias in Koch’s triangle: clinical symptoms and event recording. Europace. 2006;8:592–5. [DOI] [PubMed] [Google Scholar]
- 20.Mann DE, Kelly PA, Adler SW, Fuenzalida CE, Reiter MJ. Palpitations occur frequently following radiofrequency catheter ablation for supraventricular tachycardia, but do not predict pathway recurrence. Pacing and Clinical Electrophysiology. 1993;16:1645–9. [DOI] [PubMed] [Google Scholar]
- 21.Brembilla-Perrot B, Sellal JM, Olivier A, Manenti V, Beurrier D, de Chillou C, Villemin T, Girerd N. Recurrences of symptoms after AV node re-entrant tachycardia ablation: a clinical arrhythmia risk score to assess putative underlying cause. International Journal of Cardiology. 2015;179:292–6. [DOI] [PubMed] [Google Scholar]
- 22.Peto R Experimental survival curves for interval-censored data. Journal of the Royal Statistical Society Series C (Applied Statistics). 1973;22:86–91. [Google Scholar]
- 23.Turnbull BW. The empirical distribution function with arbitrarily grouped, censored and truncated data. Journal of the Royal Statistical Society Series B (Methodological). 1976;38:290–5. [Google Scholar]
- 24.Bryarly M, Phillips LT, Fu Q, Vernino S, Levine BD. Postural Orthostatic Tachycardia Syndrome: JACC Focus Seminar. Journal of the American College of Cardiology. 2019;73:1207–28 [DOI] [PubMed] [Google Scholar]
- 25.Hanninen M, Yeung-Lai-Wah N, Massel D, Gula LJ, Skanes AC, Yee R, Klein GJ, Manlucu J, Leong-Sit P. Cryoablation versus RF ablation for AVNRT: a meta-analysis and systematic review. Journal of Cardiovascular Electrophysiology. 2013;24:1354–60. [DOI] [PubMed] [Google Scholar]
- 26.Santangeli P, Proietti R, Di Biase L, Bai R, Natale A. Cryoablation versus radiofrequency ablation of atrioventricular nodal reentrant tachycardia. Journal of Interventional Cardiac Electrophysiology. 2014;39:111–9. [DOI] [PubMed] [Google Scholar]


