Abstract
Mycobacterium tuberculosis (MTB) complex is comprising of pathogenic mycobacteria responsible for human and animal tuberculosis, a major public health problem in Niger. Although infected individuals are paramount sources of contamination, nevertheless alternative, neglected sources may play some role in minority forms of the infection. Accordingly, we investigated the presence of Mycobacterium tuberculosis complex in soil samples in Niger. A total of 103 soil samples were collected in six different areas in Niger in October and November 2018 and April and May 2020 from residential areas of tuberculosis patients. Screening PCR targeting M. tuberculosis complex CRISPR-Csm4 and Xpert MTB/RIF Ultra assay were applied to detect the M. tuberculosis complex. M. tuberculosis DNA was positively detected in five of 103 (5/103; 4.8%) soil samples (Dosso: one sample, Zinder: one sample and Niamey: three samples) using the CRISPR-Csm4 system. CRISPR-Csm4 gene sequence identified four M. tuberculosis sensu stricto (may be lineages 1, 3 or 4) and one M. tuberculosis L2 lineage (Beijing). Moreover, the five positive samples were confirmed by Xpert MTB/RIF Ultra assay as rifampicin-susceptible M. tuberculosis complex strains. However, culture remained negative after 42 days. In this study, we announced for the first time the presence of M. tuberculosis sensu stricto in the soil of Niger. Moreover, these detected lineages were identical to the dominant M. tuberculosis lineages in patients. The presence of common lineages of M. tuberculosis between the soil and human highlight the risk of transmission from the soil to human.
Keywords: Mycobacterium tuberculosis complex, Niger, soil, tuberculosis, West Africa
Introduction
Tuberculosis is a deadly transmissible disease, which remains a major cause of death in the world despite the availability of effective chemotherapy for more than 60 years [1]. It is caused by mycobacteria forming so-called Mycobacterium tuberculosis (MTB) complex that is currently comprising ten species (Mycobacterium africanum, Mycobacterium bovis, Mycobacterium canettii, Mycobacterium caprae, Mycobacterium microti, Mycobacterium mungi, Mycobacterium orygis, Mycobacterium pinnipedii, M. tuberculosis sensu stricto and Mycobacterium suricattae) [[2], [3], [4]]. While M. tuberculosis sensu stricto is majoritarily diagnosed in human populations; nevertheless, it also could infect non-human mammals and so be implicated as a zoonotic and reverse-zoonotic pathogen [1]. Likewise, the other above-cited M. tuberculosis complex species exhibited a relative host specificity, with M. bovis responsible for zoonotic tuberculosis in the human population, being majoritarily diagnosed in cattle [5], M. caprae being primarily a pathogen of goats and sheep [6] also infecting human patients [7], Mycobacterium microti affecting voles [8,9] and rarely Human patients [10].
Zoonotic M. bovis tuberculosis supported shared efforts of veterinary and medical microbiologists to further investigate the natural cycle of transmission of this pathogen to animals and humans. For example, in West and Central Africa, there is an intermittent exposure of humans to body fluids, faeces and tissues of great apes that are constantly hunted [[11], [12], [13]] and have been demonstrated to host M. tuberculosis complex species [14,15]. In Africa also, M. tuberculosis has been reported in elephants [16,17] and birds [18], while M. tuberculosis was found in milk of cows in Ethiopia [19]. Faeces elimination of M. tuberculosis by such infected animals questioned the possibility to further detect M. tuberculosis in soil, and a recent review of experimental data and field observations confirmed the possibility of prolonged conservation of M. tuberculosis in the soil [20]. Accordingly, experimental isolation of M. tuberculosis from soil samples has been reported, and it has evidence that M. tuberculosis indeed remains viable and infectious after one year in sterilized soil in a laboratory environment was demonstrated [21].
The geographical diversity, composition and abundance of environmental mycobacteria reservoirs are not well described in Niger, despite the need to address the risk of exposure in the context of disease. The objective of this study was to further evaluate the detection of M. tuberculosis in the soil to contribute to understanding the ecology of M. tuberculosis in Niger.
Materials and methods
Sample collection and preparation
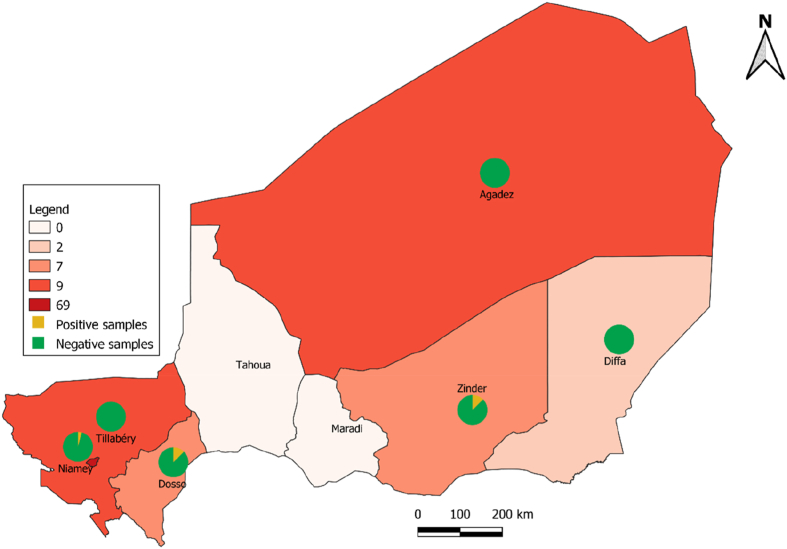
This study implying transborder transportation of environmental soil specimens collected with local authorization of Secrétariat Exécutif du Conseil National de l’Environnement pour un Développement Durable N°0153/SE/CNEDD, in Niger, towards France where further research investigations were conducted, fell order rules of the Nagoya treaty consigned by Niger and France. Accordingly, a total of 103 soil samples were collected from six regions in Niger: Agadez n = 9, Diffa n = 2, Dosso n = 7, Niamey n = 69, Tillaberi n = 10, Zinder n = 6 from October to November 2018 and April to May 2020. More precisely, 99 soil samples were collected in sites exposed to microbiologically documented pulmonary tuberculosis patients (patient living environment n = 95 and Centre National de Lutte contre la Tuberculose et des Maladies Respiratoires CNLT/MR in Niamey n = 4), and four additional samples were collected in Niamey from the piles of sand used for the building of houses to serve as negative controls. Every sample consisted of 3–10 g of soil collected at 0–5 cm depth in a sterile 50-mL tube. The samples were taken in dry places, exposed to the sun for some and for others sheltered from sunlight under trees, under a shed and in boxes. These samples were collected during the supervision visit of the health centres for the diagnosis and treatment of tuberculosis. All samples were stored at 4 °C and shipped to MEPHI, IHU Méditerranée Infection, Marseille, France, for laboratory investigations.
DNA extraction and purification
DNA extraction from soil samples was adapted from a previously described protocol [22]: 0.5 g of soil were mixed with 1 mL of lysis buffer (900 μL of 0.5 M EDTA, 10 μL of 25 mg/mL proteinase K, 90 μL nuclease-free water) into a 1.5 mL tube (Sarstedt, Nümbrecht, Germany) while water was used as a negative control. Preparations were incubated for 18 h at 37 °C on a rotative wheel, and the mixture was centrifuged for two min at 16,000 g. The clear supernatant was mixed with 10 mL of binding buffer (3.6 mL of nuclease-free water, 7.16 g of guanidine hydrochloride powder, 6 mL of isopropanol, 50 μL of freshly prepared 5% Tween-20, 450 μL of 3M sodium acetate (pH5.2) into a DNA-free 50 mL Falcon tube and DNA was purified using the MinElute Silica Spin column previously placed on a the Qiagen Vacuum (QIAGEN, Courtaboeuf, France). The MinElute column was washed with 700 μL of buffer PE and centrifuged for one min at 16,000 g then for the elution of DNA, 12.5 μL of pre-warmed nuclease-free water were added directly on the membrane of the column and incubate for three min at room temperature and centrifuged for one min at 16,000 g. This step using 12.5 μL of pre-warmed water was eventually repeated once, and the eluate contained 25 μL of the purified DNA.
PCR-based screening
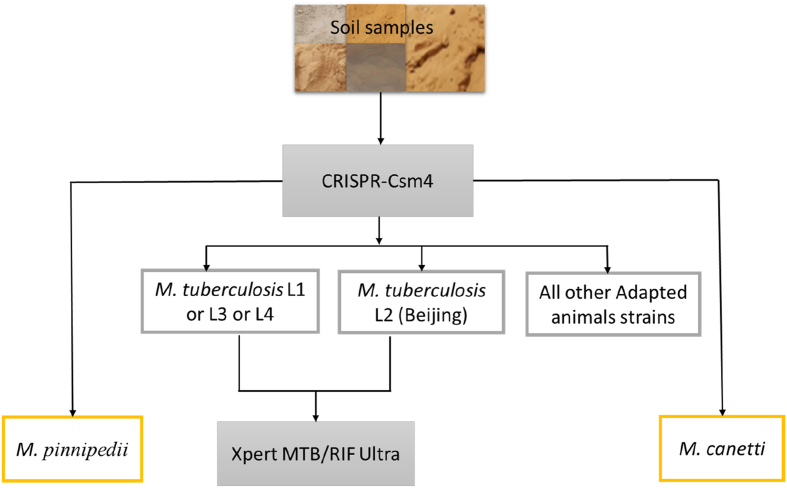
All polymerase chain reaction (PCR) experiments incorporated sterile phosphate-buffered saline (PBS) as a negative control. A home (IHU Méditerranée Infection, Marseille) designed PCR incorporating primers targeting the M. tuberculosis complex CRISPR-Csm4 gene (CRISPR type III-associated RAMP protein Csm4; gene size = 909 bp; Rv2820c) with the following sequences CRISPR-Csm4-PCRs standard (CRISPR-Csm4-ST): CRISPR-Csm4-ST1 forward, 5′-AGTCGTCCACGATTAGCTGC-3’; CRISPR-Csm4-ST1 reverse, 5′-GTAATCGGGCCCCACATAGG-3’; in order to amplify a 150-bp fragment of the M. tuberculosis complex and CRISPR-Csm4-ST2 forward, 5′-GACGCTCACGACATCCCTAC-3’; CRISPR-Csm4-ST2 reverse, 5′-GCGTAGCTGTAGACCGGATG-3’; in order to amplify a 250-bp fragment of the M. tuberculosis complex (Unpublished data). In detail, using the CRISPR-Csm4 gene allowed to differentiate M. tuberculosis (lineage 1, lineage 3 and lineage 4), M. tuberculosis Beijing lineage (L2), Mycobacterium canetti and Mycobacterium pinnipedii against all other Animal-Adapted Mycobacterium strains (Fig. 1).
Fig. 1.
CRISPR-Csm4 workflow for the detection and typing of M. tuberculosis in Niger soil.
PCR program included a 15-minute denaturation at 95 °C followed by 35 cycles at 95 °C for 30 seconds, 60 °C for 30 seconds and 72 °C for 90 seconds, followed by a final 5 min extension at 72 °C. Amplified products were visualized by 1.5% agarose gel electrophoresis. PCR products were purified and sequenced using the BigDye terminator 1.1 Cycle sequencing kit (Applied Biosystem, Courtaboeuf, France) and Genetic Analyser 3500 (Applied Bio-systems, USA).
Xpert MTB/RIF ultra assay
For that assay, 2 g of soil were mixed with 2 mL of PBS, and 1 mL of that mixture was incubated with an equal volume of magnetic beads aimed to capture the M. tuberculosis complex (Tb-Beads; Microsens Medtech Ltd., London, United Kingdom) at room temperature for two minutes as previously described [23]. After tubes were placed on the magnetic holder for exactly one minute, the supernatant was discarded, and beads were washed with one mL of 0.01 M NaOH (1/100 dilution of the NaOH 1 M), and the supernatant was quickly removed. The tubes were removed from the magnetic support, and acetic acid (pH 5.5, LABELIANS, Meyzieu, France) was added in the same volume as the starting samples, mixed and incubated at room temperature for two minutes. The tubes were placed on the magnetic support until the beads were on the wall of the tube, the supernatant was recovered (1 mL) and inactivated for one hour at 95 °C. Then, 1 mL supernatant was mixed with 1 mL of sample reagent buffer (supplied within the kit Xpert® MTB/RIF Ultra, Cepheid Europe, Maurens-Scopont, France), incubated for 15 min with occasional shaking and then added to the sample loading chamber of the Xpert® MTB/RIF Ultra Xpert’s cartridge for automatic processing into the GeneXpert instrument (Cepheid).
Culture-based method
The soil samples that have been detected positive by PCR for M. tuberculosis complex DNA along with one negative control soil specimen (avoided of any PCR-based piece of evidence of M. tuberculosis complex DNA) were further investigated by culture. These soil specimens were incubated for 12 hours at 37 °C in 5 mL in TransBK, a liquid medium designed for the decontamination and enrichment in M. tuberculosis of samples (Culture-Top, Marseille, France) with 2 mL of the suspension. Then 1 mL of the solution was incubated in the blood culture bottle with a liquid medium designed for the culture of M. tuberculosis complex in the BACT/ALERT® VIRTUO® an automat. The detection of the M. tuberculosis complex was performed every week. For detecting contamination, 50 mL of liquid culture were incubated in medium Columbia agar +5% sheep blood (bioMérieux, Marcy-l'Étoile, France) for 24 hours in an aerobic atmosphere at 37 °C. Then colonies were identified using Bruker Biotyper Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (MALDI-TOF) as previously described [24].
Mycobacterium-amoeba coculture
The samples that have been detected positive by PCRs for M. tuberculosis complex DNA along with four negative control soil specimens (avoided of any PCR-based piece of evidence of M. tuberculosis complex DNA), were further investigated by coculture with the amoeba. These soil specimens have been decontaminated as previously described [25]. The strain Acanthamoeba polyphaga LINC AP1 was used for the coculture: 2.5 mL of amoebal culture were inoculated with 100 μL of a soil suspension, and vancomycin 10 μg/mL, imipenem 10 μg/mL and voriconazole 40 μg/mL were added in 12.5 cm square flasks (SARSTEDT, Nümbrecht, Germany). The coculture was incubated in an aerobic atmosphere at 32 °C. Ziehl–Neelsen (ZN) stain for the presence of acid-fast bacilli (AFB) was performed weekly for up to 42 days.
Results
PCR-based screening
While negative controls remained negative, 4/103 (3.8%) positives samples identified by CRISPR-Csm4 sequencing as M. tuberculosis sensu stricto (may be lineage 1 or lineage 3 or lineage 4) by specific mutation A-807 in the C-terminal of the gene (gene size = 909 bp) and 1/103 (0.9%) as M. tuberculosis Beijing lineage with 552-bp large deletion fragment in the C-terminal of the gene (Fig. 1, Table 1).
Table 1.
The result of soil samples detected by CRISPR-Csm4 PCR assay
| Samples | Origin | % Identification of similarity | Identification PCRs CRISPR-Csm4-standard | genes |
|---|---|---|---|---|
| Sample DO5 | Dosso | 100% | M. tuberculosis sensu stricto (L1 or L3 or L4) | Mutation A-807 in the C-terminal |
| Sample ZD5 | Zinder | 100% | M. tuberculosis sensu stricto Beijing (L2) | Deletion fragment in the C-terminal |
| Sample NY20 | Niamey | 100% | M. tuberculosis sensu stricto (L1 or L3 or L4) | Mutation A-807 in the C-terminal |
| Sample NY36 | Niamey | 100% | M. tuberculosis sensu stricto (L1 or L3 or L4) | Mutation A-807 in the C-terminal |
| Sample NY52 | Niamey | 100% | M. tuberculosis sensu stricto (L1 or L3 or L4) | Mutation A-807 in the C-terminal |
Further, the Xpert MTB/RIF Ultra assay confirmed these five positive samples as being rifampicin susceptible (Table 2). In these two analysis steps, all negative controls remained negative (Fig. 2).
Table 2.
The result of soil samples detected by Xpert MTB/RIF Ultra PCR assay
| Samples | Origin | Identification | Cycle threshold (Ct) |
Rifampicin | |||
|---|---|---|---|---|---|---|---|
| rpoB1 | rpoB2 | rpoB3 | rpoB4 | ||||
| Negative sample 1 | Niamey | M. tuberculosis not detected | 0 | 0 | 0 | 0 | Not Applicable |
| Negative sample 2 | Niamey | M. tuberculosis not detected | 0 | 0 | 0 | 0 | Not Applicable |
| Negative sample 3 | Niamey | M. tuberculosis not detected | 0 | 0 | 0 | 0 | Not Applicable |
| Negative sample 4 | Niamey | M. tuberculosis not detected | 0 | 0 | 0 | 0 | Not Applicable |
| Sample DO5 | Dosso | M. tuberculosis detected | 32.9 | 31 | 33.8 | 37.4 | Susceptible |
| Sample ZD5 | Zinder | M. tuberculosis detected | 33.9 | 33 | 35.8 | 39.2 | Susceptible |
| Sample NY20 | Niamey | M. tuberculosis detected | 30.4 | 29.1 | 31.7 | 35.3 | Susceptible |
| Sample NY36 | Niamey | M. tuberculosis detected | 28.5 | 28.1 | 30.1 | 31.5 | Susceptible |
| Sample NY52 | Niamey | M. tuberculosis detected | 29.7 | 29 | 31.5 | 35 | Susceptible |
Fig. 2.
Geographic spread of samples in Niger and distribution of positive and negative samples in Niger. A map of the regions in Niger is shown in which pie charts indicate negative samples in green and positive samples in yellow.
Mycobacterium culture and Mycobacterium-amoeba coculture
A positive signal was detected with the blood culture bottle after seven days of incubation in an automat BACT/ALERT® VIRTUO®. The MALDI-TOF MS was performed and identified Enterobacter cloacae with a score of 2.26 (one sample in Zinder), Bacillus cereus with a score of 2.26 (control sample and two samples in Niamey), Bacillus megaterium with a score of 2.19 (one sample in Dosso) and Microbacterium aurum with a score of 1.85 (one sample in Niamey). In addition, using Mycobacterium-amoeba coculture, did not detect M. tuberculosis colony growth up to 42 days of incubation.
Discussion and conclusion
In this study, two PCR-based assays were used to detect the presence of M. tuberculosis complex DNA from soil samples collected in sites exposed to tuberculosis patients in Niger. Among 103 soil samples, five positive soil samples were detected. Culture attempts, however, remained negative, mainly due to contamination of the culture media, leaving unknown whether such finally detected M. tuberculosis was alive or dead.
In this study, detected lineages were known as predominant lineages in tuberculosis patients in Niger; and have been already detected in soil and water in Tehran, Iran [26]. In addition, the isolation of M. tuberculosis from soil samples has been reported in a Russian study [27]. Experimental observation in a laboratory environment showed an evidence that M. tuberculosis indeed remains viable and infectious after one year in sterilized soil [21]. On the other hand, several reports have indicated that M. bovis in the M. tuberculosis complex is able to survive in soil and has been shown to survive in, outside a host, for substantial periods of time under favourable conditions the environment [[28], [29], [30], [31], [32]].
In detail, Nigerien patients with pulmonary tuberculosis may spit into the floor. Some inhabitants emit defecation in the ground; it is done in different ways: by squatting in soil or using the traditional outdoor toilets. Tuberculosis patient stools may be, in Niger, a source for soil contamination by M. tuberculosis. Indeed, studies have shown that M. tuberculosis can be found in the stool of patients with pulmonary tuberculosis and can be used for the diagnosis of tuberculosis by culture [33] and by molecular detection [34]. However, the role of soil as a source of contamination of certain mammals was possible. In addition, there has been a case of transmission of M. tuberculosis to an embalmer from the cadaver of a patient who died of pulmonary tuberculosis [35], illustrating that buried corpses of tuberculosis patients could be a source of soil infection [1]. A subsequent study showed that M. tuberculosis organisms remain viable and therefore infectious for at least 24 to 48 hours after an infected cadaver has been embalmed [36].
As a perspective about this study, we need to isolate these positive samples in order to recover the complete genome sequence. Genome sequences were required to compare these samples with other human M. tuberculosis genomes strains in Niger to confirm the transmission of M. tuberculosis between soil and patient.
Authors’ contributions
All authors contributed to this work.
Funding
This study was supported by Fondation Méditerranée Infection, Marseille, France.
Transparency declaration
The authors have no conflicts of interest to declare.
Acknowledgements
The authors would like to thank the staff of the national reference laboratory for TB (Niamey), the Programme National de Lutte contre la Tuberculose (PNLT), Niamey and the staff of the Centre National de Lutte contre la Tuberculose et des Maladies Respiratoires (CNLT/MR), Niamey.
References
- 1.Ghodbane R., Drancourt M. Non-human sources of Mycobacterium tuberculosis. Tuberculosis. 2013;93:589–595. doi: 10.1016/j.tube.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Parsons S.D.C., Drewe J.A., Gey van Pittius N.C., Warren R.M., van Helden P.D. Novel cause of tuberculosis in meerkats, South Africa. Emerg Infect Dis. 2013;19:2004–2007. doi: 10.3201/eid1912.130268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Ingen J., Rahim Z., Mulder A., Boeree M.J., Simeone R., Brosch R. Characterization of Mycobacterium orygis as M. tuberculosis complex subspecies. Emerg Infect Dis. 2012;18:653–655. doi: 10.3201/eid1804.110888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander K.A., Laver P.N., Michel A.L., Williams M., van Helden P.D., Warren R.M. Novel Mycobacterium tuberculosis complex pathogen. M Mungi Emerg Infect Dis. 2010;16:1296–1299. doi: 10.3201/eid1608.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ocepek M., Pate M., Žolnir-Dovč M., Poljak M. Transmission of Mycobacterium tuberculosis from human to cattle. J Clin Microbiol. 2005;43:3555–3557. doi: 10.1128/JCM.43.7.3555-3557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aranaz A., Liébana E., Gómez-Mampaso E., Galán J.C., Cousins D., Ortega A. Mycobacterium tuberculosis subsp. caprae subsp. nov.: a taxonomic study of a new member of the Mycobacterium tuberculosis complex isolated from goats in Spain. Int J Syst Evol Microbiol. 1999;49:1263–1273. doi: 10.1099/00207713-49-3-1263. [DOI] [PubMed] [Google Scholar]
- 7.Yeboah-Manu D., Asare P., Asante-Poku A., Otchere I.D., Osei-Wusu S., Danso E. Spatio-temporal distribution of Mycobacterium tuberculosis complex strains in Ghana. PLoS One. 2016;11 doi: 10.1371/journal.pone.0161892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wells A.Q. Tuberculosis in wild voles. Lancet. 1937;229:1221. doi: 10.1016/S0140-6736(00)83505-9. [DOI] [Google Scholar]
- 9.Frota C.C., Hunt D.M., Buxton R.S., Rickman L., Hinds J., Kremer K. Genome structure in the vole bacillus, Mycobacterium microti, a member of the Mycobacterium tuberculosis complex with a low virulence for humans. Microbiology. 2004;150:1519–1527. doi: 10.1099/mic.0.26660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguga-Phasha N.T.C., Munyai N.S., Mashinya F., Makgatho M.E., Mbajiorgu E.F. Genetic diversity and distribution of Mycobacterium tuberculosis genotypes in Limpopo, South Africa. BMC Infect Dis. 2017;17:764. doi: 10.1186/s12879-017-2881-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruse H., Kirkemo A.-M., Handeland K. Wildlife as source of zoonotic infections. Emerg Infect Dis. 2004;10:2067–2072. doi: 10.3201/eid1012.040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bennett E.L., Blencowe E., Brandon K., Brown D., Burn R.W., Cowlishaw G. Hunting for consensus: reconciling bushmeat harvest, conservation, and development policy in West and central Africa. Conservat Biol. 2007;21:884–887. doi: 10.1111/j.1523-1739.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 13.Coscolla M., Lewin A., Metzger S., Maetz-Rennsing K., Calvignac-Spencer S., Nitsche A. Novel Mycobacterium tuberculosis complex isolate from a wild chimpanzee. Emerg Infect Dis. 2013;19:969–976. doi: 10.3201/eid1906.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Une Y., Mori T. Tuberculosis as a zoonosis from a veterinary perspective. Comp Immunol Microbiol Infect Dis. 2007;30:415–425. doi: 10.1016/j.cimid.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Shipley S.T., Coksaygan T., Johnson D.K., McLeod C.G., DeTolla L.J. Diagnosis and prevention of dissemination of tuberculosis in a recently imported rhesus macaque (Macaca mulatta) J Med Primatol. 2008;37:20–24. doi: 10.1111/j.1600-0684.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- 16.Mikota S.K., Peddie L., Peddie J., Isaza R., Dunker F., West G. Epidemiology and diagnosis of Mycobacterium tuberculosis in captive Asian elephants (Elephas maximus) J Zoo Wildl Med. 2001;32:1–16. doi: 10.1638/1042-7260(2001)032[0001:EADOMT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Obanda V., Poghon J., Yongo M., Mulei I., Ngotho M., Waititu K. First reported case of fatal tuberculosis in a wild African elephant with past human–wildlife contact. Epidemiol Infect. 2013;141:1476–1480. doi: 10.1017/S0950268813000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovalev G.K. Tuberculosis in wildlife (review) https://scholar.google.com/scholar?hl=en&as_sdt=0%2C5&q=Kovalev+GK.+Tuberculosis+in+wildlife+%28review%29.+J+Hyg+Epidemiol+Microbiol+Immunol+1980%3B24%3A495e504.&btnG= J... - Google Scholar n.d. [PubMed]
- 19.Fetene T., Kebede N., Alem G. Tuberculosis infection in animal and human populations in three districts of western gojam, Ethiopia: tuberculosis infection in animal and human populations in three districts. Zoonoses Publ Health. 2011;58:47–53. doi: 10.1111/j.1863-2378.2009.01265.x. [DOI] [PubMed] [Google Scholar]
- 20.Martinez L., Verma R., Croda J., Horsburgh C.R., Walter K.S., Degner N. Detection, survival and infectious potential of Mycobacterium tuberculosis in the environment: a review of the evidence and epidemiological implications. Eur Respir J. 2019;53:1802302. doi: 10.1183/13993003.02302-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghodbane R., Medie F.M., Lepidi H., Nappez C., Drancourt M. Long-term survival of tuberculosis complex mycobacteria in soil. Microbiology. 2014;160:496–501. doi: 10.1099/mic.0.073379-0. [DOI] [PubMed] [Google Scholar]
- 22.Spyrou M.A., Keller M., Tukhbatova R.I., Scheib C.L., Nelson E.A., Andrades Valtueña A. Phylogeography of the second plague pandemic revealed through analysis of historical Yersinia pestis genomes. Nat Commun. 2019;10:4470. doi: 10.1038/s41467-019-12154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saad J., Loukil A., Drancourt M. Bead-captured Mycobacterium tuberculosis for next-generation sequencing diagnosis of uncultured tuberculosis. Eur J Clin Microbiol Infect Dis. 2020;39:205–207. doi: 10.1007/s10096-019-03700-1. [DOI] [PubMed] [Google Scholar]
- 24.Patel R. MALDI-TOF MS for the diagnosis of infectious diseases. Clin Chem. 2015;61:100–111. doi: 10.1373/clinchem.2014.221770. [DOI] [PubMed] [Google Scholar]
- 25.Asmar S., Drancourt M. Chlorhexidine decontamination of sputum for culturing Mycobacterium tuberculosis. BMC Microbiol. 2015;15:155. doi: 10.1186/s12866-015-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Velayati A.A., Farnia P., Mozafari M., Malekshahian D., Farahbod A.M., Seif S. Identification and genotyping of Mycobacterium tuberculosis isolated from water and soil samples of a metropolitan city. Chest. 2015;147:1094–1102. doi: 10.1378/chest.14-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozlov V.S., Rotov V.I. Isolation of Mycobacterium tuberculosis cultures from soil. Probl Tuberk. 1977:78–80. [PubMed] [Google Scholar]
- 28.Duffield B.J., Young D.A. Survival of Mycobacterium bovis in defined environmental conditions. Vet Microbiol. 1985;10:193–197. doi: 10.1016/0378-1135(85)90021-5. [DOI] [PubMed] [Google Scholar]
- 29.Jackson R., de Lisle G.W., Morris R.S. A study of the environmental survival of Mycobacterium bovis on a farm in New Zealand. New Zealand Vet J. 1995;43:346–352. doi: 10.1080/00480169./1995.35918. [DOI] [PubMed] [Google Scholar]
- 30.Tanner M., Michel A.L. Investigation of the viability of M. bovis under different environmental conditions in the Kruger National Park. Onderstepoort J Vet Res. 1999;66:185–190. PMID: 10631708. [PubMed] [Google Scholar]
- 31.Williams R.S., Hoy W.A. The viability of B. Tuberculosis (bovinus) on pasture land, in stored faeces and in liquid manure. Epidemiol Infect. 1930;30:413–419. doi: 10.1017/S0022172400010561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddock E.C.G. Studies on the survival time of the bovine tubercle Bacillus in soil, soil and dung, in dung and on grass, with experiments on the preliminary treatment of infected organic matter and the cultivation of the organism. Epidemiol Infect. 1933;33:103–117. doi: 10.1017/S002217240001843X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asmar S., Chatellier S., Mirande C., van Belkum A., Canard I., Raoult D. A novel solid medium for culturing Mycobacterium tuberculosis isolates from clinical specimens. J Clin Microbiol. 2015;53:2566–2569. doi: 10.1128/JCM.01149-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ElKhéchine A., Henry M., Raoult D., Drancourt M. Detection of Mycobacterium tuberculosis complex organisms in the stools of patients with pulmonary tuberculosis. Microbiology (Reading, England) 2009;155:2384–2389. doi: 10.1099/mic.0.026484-0. [DOI] [PubMed] [Google Scholar]
- 35.Sterling T.R., Pope D.S., Bishai W.R., Harrington S., Gershon R.R., Chaisson R.E. Transmission of Mycobacterium tuberculosis from a cadaver to an embalmer. N Engl J Med. 2000;342:246–248. doi: 10.1056/NEJM200001273420404. [DOI] [PubMed] [Google Scholar]
- 36.Weed L.A., Baggenstoss A.H. The isolation of pathogens from tissues of embalmed human bodies. Am J Clin Pathol. 1951;21:1114–1120. doi: 10.1093/ajcp/21.12.1114. [DOI] [PubMed] [Google Scholar]