Figure 9.
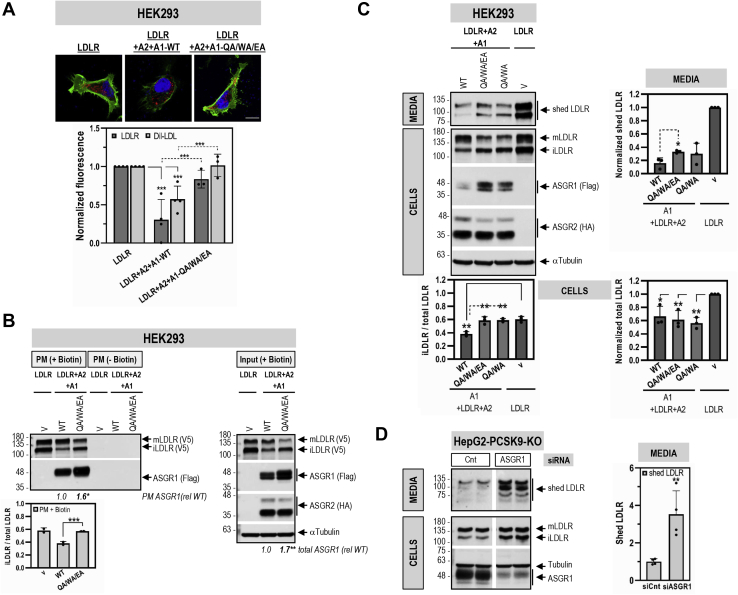
The lectin-binding domain in ASGR1 is important for the ASGR1-mediated degradation of the functional, mainly non-O-glycosylated, LDLR at the plasma membrane. HEK293 cells were transfected with V5-tagged LDLR alone or in combination with HA-tagged ASGR2 and Flag-tagged ASGR1, WT or its carbohydrate-binding mutants Q240A/W244A/E253A (QA/WA/EA) (A–C) or Q240A/W244A (QA/WA) (C). A, immunofluorescence microscopy of plasma membrane–overexpressed LDLR (green signal) and DiI-LDL uptake (red signal) for 2 h at 37 °C before fixation. Nontransfected cells (V) expressed ∼100-fold less LDLR than transfected ones. The scale bar represents 15 μm. B, surface protein biotinylation: cells incubated with Biotin (+Biotin) or PBS (−Biotin) were lysed and plasma membrane (PM) proteins were pulled down with streptavidin and analyzed by Western blot (WB) for LDLR (V5-HRP) and ASGR1 (Flag-HRP) (left panel). One-eighth (12.5%; 30 μg) of the (+Biotin) cell lysate before streptavidin pull-down (input) was analyzed by WB (right panel). C, WB analyses of total cellular LDLR (mature mLDLR and immature iLDLR), ASGR1 (Flag-HRP) and ASGR2 (HA-HRP), and shed LDLR in the media. The WB of the control vector condition (V) is the same as that in Figure 7. This is justified since the above control data are derived from the same experiment testing the effects on LDLR of ASGR2 alone (Fig. 7) and of ASGR1 in the presence (Figs. 7 and 9C) or absence of ASGR2 (Fig. 7). D, HepG2-PCSK9-KO cells were transfected for 48 h with 20 nM nontargeting (Cnt) siRNA or siRNA ASGR1. WB analyses of total cellular LDLR (mLDLR and iLDLR), ASGR1, and of shed LDLR in the media are shown. Note that 13% of total media and 8% of total lysates are loaded on the gel and that the exposure times are 15 and 3 min, respectively. Data are representative of at least two independent experiments. Quantifications are averages ±SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 (t test).