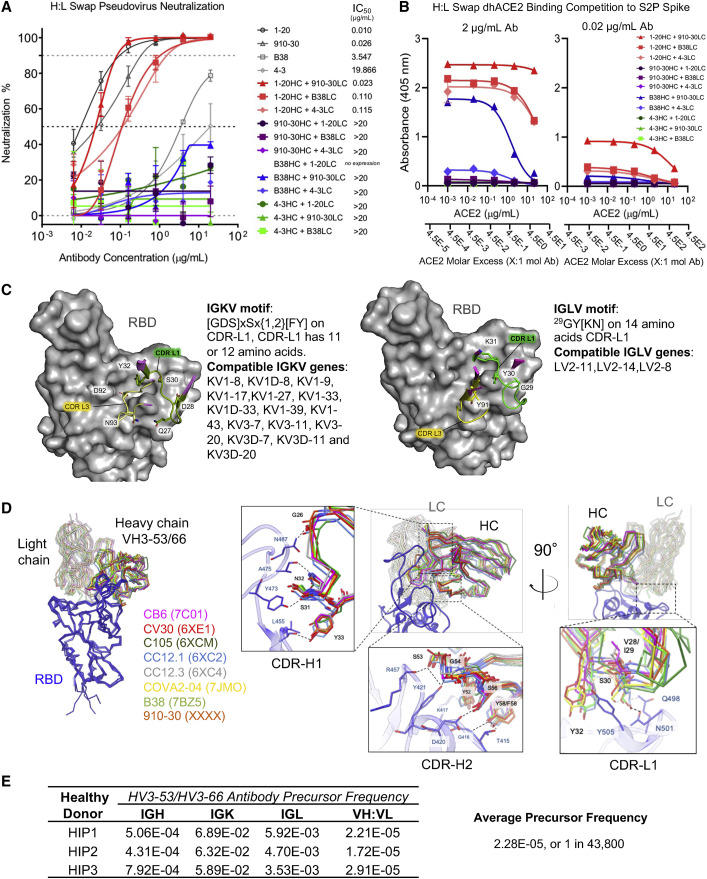
Figure 3.
Heavy- and light-chain analyses reveal critical contributions of both VH and VL for potent antibody neutralization in the IGHV3-53/3-66 antibody class
(A) SARS-CoV-2 pseudovirus neutralization results for heavy-light swapped IgG panel. Data are represented as means ± SDs.
(B) dhACE2 competition ELISA against SARS-CoV-2 S2P protein showing heavy-light swapped IgG binding response to increasing dhACE2 (ACE2) concentrations. dhACE2 concentration is provided in μg/mL and also as ACE2 molar excess units. Data are represented as means ± SDs.
(C) Conserved IGHV3-53/3-66 class light-chain kappa (left panel) and lambda (right panel) genetic elements associated with contacting the RBD interface. CDR-L1 residues are not specific to IGKV1-33 (910-30) and IGLV2-8 (C105) (Table S4).
(D) Left panel: structural superposition of IGHV3-53/3-66 Fab variable domains in complex with RBD shows the same binding orientation for 8 different class antibodies aligned on RBD. Right panel: close-up views of the Fab:RBD interface for the 8 IGHV3-53/3-66 antibodies superimposed on RBD. Conserved interactions of CDR-H1, -H2, and -L1 define the structural signatures responsible for viral neutralization by the IGHV3-53/3-66 antibody class.
(E) Estimated probability of IGHV3-53/3-66 class pre-cursor antibodies derived from healthy donor (HIP1, HIP2, HIP3) immune repertories.