Key Points
Question
Among patients with acute ischemic stroke and large vessel occlusion, does an initial thrombectomy technique consisting of contact aspiration and stent retriever combined improve the reperfusion rate compared with stent retriever alone?
Findings
In this randomized clinical trial that included 408 patients, the rate of near-total or total reperfusion on the extended Thrombolysis in Cerebral Infarction scale of 2c or 3 (eTICI 2c/3) at the end of the endovascular procedure was not significantly different in the combined contact aspiration and stent retriever group compared with the stent retriever alone group (64.5% vs 57.9%; adjusted odds ratio, 1.33).
Meaning
Among patients with acute ischemic stroke due to large vessel occlusion, an initial thrombectomy technique consisting of contact aspiration and stent retriever combined did not significantly improve the rate of near-total or total (eTICI 2c/3) reperfusion at the end of the endovascular procedure as compared with stent retriever alone.
Abstract
Importance
Mechanical thrombectomy using a stent retriever or contact aspiration is widely used for treatment of patients with acute ischemic stroke due to anterior circulation large vessel occlusion, but the additional benefit of combining contact aspiration with stent retriever is uncertain.
Objective
To determine whether mechanical thrombectomy for treatment of anterior circulation large vessel occlusion stroke with initial contact aspiration and stent retriever combined results in better final angiographic outcome than with standard stent retriever alone.
Design, Setting, and Participants
This trial was a multicenter randomized, open-label, blinded end point evaluation that enrolled 408 patients from October 16, 2017, to May 29, 2018, in 11 French comprehensive stroke centers, with a 12-month outcome follow-up. Patients with a large vessel occlusion in the anterior circulation were included up to 8 hours after symptom onset. The final date of follow-up was June, 19, 2019.
Interventions
Patients were randomly assigned (1:1 allocation) to receive initial thrombectomy with contact aspiration and stent retriever combined (205) or stent retriever alone (203).
Main Outcomes and Measures
The primary outcome was the rate of expanded Thrombolysis In Cerebral Infarction score of 2c or 3 (eTICI 2c/3; ie, scores indicate near-total and total reperfusion grades) at the end of the procedure.
Results
Among the 408 patients who were randomized, 3 were excluded, and 405 (99.3%) patients (mean age, 73 years; 220 [54%] women and 185 [46%] men) were included in the primary analysis. The rate of eTICI 2c/3 at the end of the endovascular procedure was not significantly different between the 2 thrombectomy groups (64.5% [131 of 203 patients] for contact aspiration and stent retriever combined vs 57.9% [117 of 202 patients] for stent retriever alone; risk difference, 6.6% [95% CI, –3.0% to 16.2%]; adjusted odds ratio [OR], 1.33 [95% CI, 0.88 to 1.99]; P = .17). Of 14 prespecified secondary efficacy end points, 12 showed no significant difference. A higher rate of successful reperfusion was achieved in the contact aspiration combined with stent retriever group vs the stent retriever alone group (eTICI 2b50/2c/3, 86.2% vs 72.3%; adjusted OR, 2.54 [95% CI, 1.51 to 4.28]; P < .001) and of near-total or total reperfusion (eTICI 2c/3, 59.6% vs 49.5%; adjusted OR, 1.52 [95% CI, 1.02 to 2.27]; P = .04) after the assigned initial intervention alone.
Conclusions and Relevance
Among patients with acute ischemic stroke due to large vessel occlusion, an initial thrombectomy technique consisting of contact aspiration and stent retriever combined, compared with stent retriever alone, did not significantly improve the rate of near-total or total reperfusion (eTICI 2c/3) at the end of the endovascular procedure, although the trial may have been underpowered to detect smaller differences between groups.
Trial Registration
ClinicalTrials.gov Identifier: NCT03290885
This randomized clinical trial compares the effect of thrombectomy with contact aspiration and stent retriever combined vs stent retriever alone on revascularization in patients with acute ischemic stroke and large vessel occlusion.
Introduction
Several randomized clinical trials (RCTs)1 have demonstrated that endovascular mechanical thrombectomy leads to a better functional outcome compared with medical therapy in patients with acute ischemic stroke due to large vessel occlusion.
Mechanical thrombectomy strategies encompass the use of second-generation devices such as stent retrievers or contact aspiration catheters as an initial therapy for stroke.1,2 Reperfusion is a strong predictor of clinical outcome and, to date, whatever the technique used as the initial approach (contact aspiration or stent retriever thrombectomy), the rate of good reperfusion, corresponding with a total score of 3 on the expanded Thrombolysis In Cerebral Infarction scale (eTICI 3; score indicates complete perfusion) or near-total (eTICI 2c; score indicates near-complete perfusion ) reperfusion scores, remains below 60%.2,3,4 Both European and US recommendations propose that clinical trials be conducted to determine the best strategy of mechanical thrombectomy device uses that achieve the highest rate of successful reperfusion.5,6
The concomitant use of contact aspiration during stent retriever thrombectomy (combination of contact aspiration and stent retriever) has been proposed as a more efficient technique to achieve a higher rate of reperfusion due to the potential synergistic effect of the 2 techniques used simultaneously.7,8,9,10 Nevertheless, this potential synergistic effect of contact aspiration and stent retriever devices in initial mechanical thrombectomy remains uncertain and needs to be evaluated in a head-to-head RCT. This present RCT aimed to test whether combining contact aspiration and stent retriever techniques improves the rate of eTICI 2c/3 reperfusion at the end of the procedure when compared with stent retriever alone as an initial thrombectomy strategy in patients undergoing endovascular treatment for large vessel occlusion.
Methods
According to French laws, oral informed consent was sought from patients if their level of consciousness was sufficient or from a relative. This study operated under an emergency inclusion protocol due to the nature of the condition. When the patient regained a satisfactory state of consciousness, consent was requested for pursuit of the research and treatment based on the information collected.
The study protocol and the consent form were approved by the Comité de Protection des Personnes (CPP) Ile de France VI (ID 2016-A01735-46). The details of the trial protocol (published previously11) and the statistical analysis plan have been included in Supplement 1 and Supplement 2.
Trial Design
This trial was a randomized, multicenter, clinical, open-label, blinded end point trial. The trial was designed to compare the results obtained with the use of contact aspiration and stent retriever combined (experimental intervention group) vs use of stent retriever alone (control group). Patients were recruited in 11 high-volume, comprehensive stroke centers in France, all of which regularly perform both contact aspiration and stent retriever techniques. High-volume centers were those that carried out at least 100 thrombectomies per year and where all physicians had already performed more than 10 endovascular procedures with stent retriever and contact aspiration techniques. Screening and enrollment into this study started in October 2017 and was completed in June 2018. Patients who fulfilled the entry criteria were randomized. The data collection phase of the study was completed by October 2018 for the 3-month follow-up and by June 2019 for the 1-year follow-up.
Data collection during the study was carried out using an electronic case report form, developed using Clinsight software (Publisher ENNOV).
Patient Population
This study enrolled adults (no upper age limit) admitted with suspected acute ischemic stroke due to a large vessel occlusion in the anterior circulation, within 8 hours of symptom onset.
Exclusion criteria included acute ischemic stroke involving posterior circulation, known or suspected preexisting (chronic) large vessel occlusion in the symptomatic territory, angiographic evidence of tandem cervical occlusion or stenosis requiring treatment, allergy to radiographic contrast agents, disability prior to stroke occurrence (modified Rankin Scale score >3), pregnant or breastfeeding women, severe or fatal comorbidities that would likely prevent improvement or follow-up or that would render the patient unlikely to benefit from the procedure, patient being under legal protection, no affiliation with a social security scheme, opposition from the patient or the family.
Randomization
Immediately following baseline brain imaging and prior to endovascular thrombectomy, patients were randomly allocated in a 1:1 ratio to receive, as the initial intervention, thrombectomy by the combined contact aspiration and stent retriever technique (experimental intervention group) orby the standard stent retriever technique alone (control group) (Figure 1). A dynamic randomization procedure using the Pocock and Simon minimization method12 was used to achieve a balance of the following factors related to the final eTICI score at the end of the endovascular procedure: age (≤70 vs >70 years), prior use of intravenous thrombolysis, and occlusion site (isolated middle cerebral artery vs terminus internal carotid artery). The center was also considered in the minimization method. The dynamic randomization procedure was performed using the electronic case report form developed with Clinsight software (Castor). The first participant was allocated to the treatment group at random, and for each subsequent participant, an imbalance score was computed from the stratification variables and was used to determine which treatment would lead to better balance between the groups in the stratification variables. In the case of equal scores, the participant was allocated to the treatment group at random, and in the case of unequal scores, the participant with a lower score was allocated to the treatment group with a lower score. Vascular neurologists or neurointerventionalists carried out patient enrollment and randomization. Randomization numbers were allocated sequentially in the order of patient inclusion in the trial.
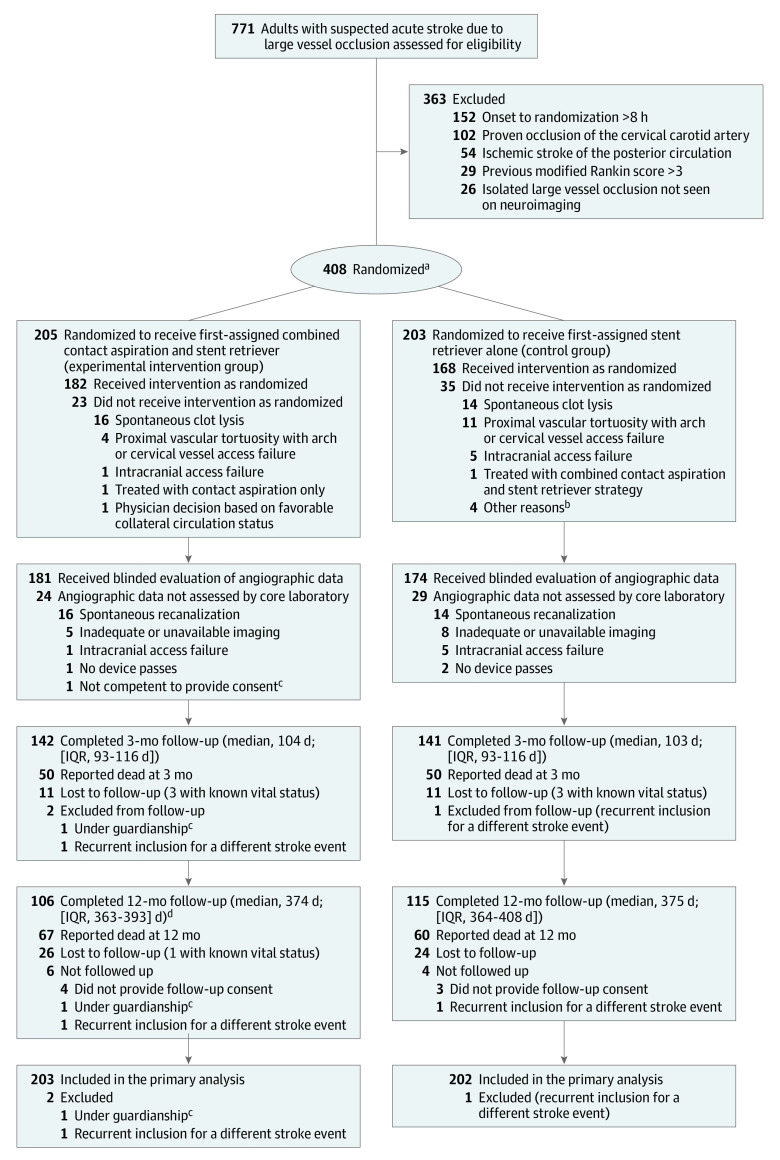
Figure 1. Flowchart of Participation in the ASTER2 Trial.
aRandomization by minimization was implemented to ensure a balance between treatment groups on the variables of center and patients’ age (≤70 vs >70 years), prior use of intravenous thrombolysis, and occlusion site (isolated middle cerebral artery vs others).
bReasons: 1 patient was transferred to another hospital before thrombectomy, 1 was not treated using a balloon-guide catheter (device unavailable), 1 was not treated due to hemorrhagic transformation before groin puncture, and 1 was treated with aspiration by guiding catheter.
cDeferred consent could not be obtained for 1 patient because the patient was not competent and no legally authorized representative was available to provide proxy consent.
dIncludes 1 death that occurred after 12-month follow-up (at 451 days).
Interventions and Thrombectomy Procedure
According to the recommendations of the American Stroke Association and the European Stroke Organisation,6,13 patients with an acute proximal artery occlusion within 8 hours of symptom onset (or <8 hours of when last seen well) were given intravenous thrombolysis (if eligible and within the 4.5 hours after stroke onset or based on a mismatch between positive diffusion-weighted imaging and negative fluid-attenuated inversion recovery imaging) and were rapidly transferred to the angiographic suite if they fulfilled the inclusion criteria and presented none of the exclusion criteria. After endovascular treatment, the patients were transferred to the stroke unit to receive usual care.
The assigned endovascular strategy was carried out using general anesthesia, conscious sedation, or local anesthesia, according to the local practice. Arterial puncture to initiate the assigned procedure was performed within 1 hour of randomization. An introducer sheath was placed in the femoral artery, and diagnostic angiography was initially performed via the transfemoral approach with catheterization of the carotid artery on the side appropriate to the patient’s symptoms. As per local standard of care and prior to thrombectomy, digital subtraction angiography was performed to define the angiographic architecture of the occluded vascular segment. When possible, an assessment of collateral blood flow by digital subtraction angiography was made as per the institutional standard of care, particularly in cases of terminal internal carotid artery occlusion. Prior to mechanical thrombectomy, baseline eTICI and artery occlusion lesion scores were obtained by digital subtraction angiography (section 6.3.5 in Supplement 1). In both assigned endovascular techniques, the choice of the device was left to the operator’s discretion.
The use of a balloon-guide catheter was mandatory in both groups. The distal two-thirds of the stent retriever was deployed posterior to the clot, leaving the proximal third within the clot. Stent retriever placement was mostly in the inferior division of the middle cerebral artery because of the straighter course and larger diameter of the inferior M2 segment division. The pull-out maneuver was performed after the temporary inflation of the balloon at the tip of the balloon-guide catheter to ensure carotid flow arrest. All CE-marked contact aspiration catheters and stent retriever devices were permitted in the study (CE indicating European conformity: meeting European Union safety, health, and environmental protection requirements). Physicians were required to perform at least 3 attempts at revascularization using the assigned endovascular technique before switching to another endovascular procedure (rescue therapy) if needed and in accordance with good practice recommendations. The eTICI score was recorded after each attempt. The decisions of whether to use a rescue therapy and which technique to use were left to the discretion of the operator. Permitted rescue techniques were contact aspiration, stent retriever, combined contact aspiration and stent retriever, and angioplasty with or without stenting. Immediate posttreatment angiograms were obtained in the anterior, posterior, lateral, and working positions. Investigators were instructed to take necessary steps to ensure that preplacement and postplacement angiograms were performed using similar views, magnifications, and contrast amounts to ensure valid before vs after comparisons. The final eTICI scores were assessed after completion of the procedure. Standardization of postprocedure medical management in both groups was carried out according to European guidelines.13,14 For further details regarding the combined contact aspiration and stent retriever technique (8,9,10,15) and the stent retriever alone technique, see the eMethods section in Supplement 3.
Neurological and functional examinations were conducted using the National Institutes of Health Stroke Scale (NIHSS) and established within a mean (SD) of 24 (12) hours of randomization. Follow-up imaging (noncontrast computed tomography [CT] or magnetic resonance imaging [MRI]) was performed at 24 (12) hours after randomization and reviewed to assess hemorrhagic transformation. Regions of hemorrhagic transformation were categorized as petechial hemorrhage or hematoma according to the European Cooperative Acute Stroke Study III classification.16 In addition, any neurological deterioration was evaluated by noncontrast CT and/or other evaluations, as indicated, according to the investigator or hospital best practice. Symptomatic intracranial hemorrhage was defined as 24-hour CT or MRI evidence of an European Cooperative Acute Stroke Study–defined intracerebral hemorrhage, associated with a 4-point or more worsening of the NIHSS score.
A follow-up examination was performed at 90 days to assess the modified Rankin Scale (mRS) score and at 12 months to assess the mRS and the quality-of-life score (EuroQol EQ-5D-3L).17 Follow-up examinations were performed by trained research nurses during face-to-face interviews or via telephone survey.
Outcomes
Primary Outcome
The primary outcome was defined as the percentage of patients with an eTICI score of 2c (near-total perfusion except for slow flow in a few distal cortical vessels or presence of small distal cortical emboli) or 3 (total perfusion with normal filling of all distal branches) on digital subtraction angiography performed at the end of the endovascular procedure (ie, after the assigned treatment and any further treatment deemed necessary). eTICI scores range from grade 0 (no perfusion or anterograde flow beyond site of occlusion) to grade 3 (total reperfusion with normal filling). eTICI 2c/3 reperfusion was chosen4 as the primary outcome since it is the surrogate marker (an angiographic efficacy outcome) strongly associated with disability reduction.4,18,19
Secondary Outcomes
Secondary angiographic efficacy outcomes were reperfusion (eTICI 2b50/2c/3 and eTICI 3 at the end of the endovascular procedure), eTICI 2b50/2c/3, eTICI 2c/3 and eTICI 3 after the assigned first-allocated endovascular treatment, first-pass effect (TICI 2c/3 after first device pass), and total first pass effect (eTICI 3 after first device pass); the time from arterial puncture to achievement of eTICI 3 reperfusion and the time from clot contact to maximal reperfusion. Post hoc secondary angiographic outcome was use of rescue treatment.
Secondary clinical efficacy outcomes were disability, as assessed by the overall distribution of the mRS score (shift analysis combining scores of 5 and 6 on a 6-point disability scale; score range, 0 [no neurological symptoms] to 5 [severe disability], with a separate category of 6 [death]) at 90 days and 12 months, 90-day and 12-month favorable functional outcome as defined by an mRS score of 0 to 2, the change in NIHSS score at 24 hours, and 12-month EQ-5D scores (utility index score and visual analog scale). Post hoc secondary clinical outcome was excellent functional outcome as defined by a mRS score of 0 to 1 at 90 days and 12 months.
Safety outcomes were symptomatic and asymptomatic intracerebral hemorrhage on brain imaging (MRI or noncontrast CT) at 24 (12) hours after mechanical thrombectomy according to the European Cooperative Acute Stroke Study III classification, parenchymal hematoma type 2, all-cause mortality at 90 days and 12 months, and procedure-related serious adverse events (arterial perforation, arterial dissection, embolization in a new territory, and subarachnoid hemorrhage, spasms).
Blinding
For the primary outcome, 2 independent assessors at a central imaging core laboratory (not involved in patient management) determined the eTICI score and collateral status. In cases of disagreement between the 2 assessors, a centralized neurointerventionalist reviewed angiograms and decided on the primary end point value. All neuroimaging readings, including determination of site of arterial occlusion, clot burden score, and hemorrhagic transformation were performed by the imaging core laboratory, which was also blinded to procedure allocation. Serious adverse events and procedure-related complications were adjudicated by 3 members of the data and safety monitoring board, blinded to the treatment group. The 90-day and 1-year mRS scores were algorithmically based on answers provided by patients or their caregiver in a brief structured interview by a research nurse (blinded to treatment group allocation) during face-to-face interviews or via telephone using a validated tool.20 The NIHSS score at 24 hours was assessed by the treating physician (not blinded to the treatment group allocation).
Statistical Analysis
The study plan was to randomize a total 408 patients (204 per group) to have a statistical power of 80% to demonstrate (using a 2-sided z test with pooled variables at the 0.05 significance level) the superiority of thrombectomy with first-allocated contact aspiration and stent retriever combined over first-allocated stent retriever alone in achieving the eTICI 2c/3 reperfusion (primary outcome) at the end of the endovascular procedure. The sample size was calculated based on an expected primary outcome rate of 55% in patients treated with first-allocated stent retriever strategy,3,4 assuming an absolute increase in 15% of patients with the first-allocated combined contact aspiration and stent retriever strategy (yielding a rate of 70%),9,10 and expecting a rate of spontaneous reperfusion before mechanical thrombectomy and catheterization failures of 20% in both groups. Final analysis was conducted in an unblinded manner by an academic statistician. The primary outcome (eTICI 2c/3 reperfusion grades at the end of the endovascular procedure) and all secondary binary outcomes were compared between the 2 groups using a mixed logistic regression model adjusting for the randomization stratification variables by including age (≤70 vs >70 years), intravenous thrombolysis, and occlusion site (isolated middle cerebral artery vs terminus internal carotid artery) as fixed effects and center as a random effect (as recommended in the literature).
From this model, an effect size measure was derived: adjusted odds ratio (OR) and 95% CI for the first-allocated combined contact aspiration and stent retriever group relative to the initial stent retriever alone group. We also calculated absolute risk differences from the marginal probabilities of total and subtotal revascularization, estimated by the mixed logistic regression model using the method described by Austin.21
The secondary outcomes were analyzed using a mixed ordinal logistic regression model for ordinal outcomes (distribution of the mRS score at 3 and 12 months after combining scores of 5 and 6), a constrained longitudinal data analysis model for the change in NIHSS score from admission to 24 hours, mixed linear regression models for time from arterial puncture to eTICI 2c/3 reperfusion, time from clot contact to maximal reperfusion, EQ-5D scores at 12 months, and a frailty model (Cox proportional hazard regression) for 12-month all-cause mortality. Residual normality of a constrained longitudinal data analysis model and a linear mixed-regression model was checked graphically and satisfied for all outcomes (after applying a log-transformation for time outcomes; see eFigure 3 in Supplement 3), and proportional hazard assumption of the Cox regression model was established for the treatment group by a visual inspection of Schoenfeld residuals plot (see eFigure 4 in Supplement 3). A sensitivity analysis for primary and secondary efficacy outcomes was conducted in the per-protocol population, defined as all patients who received at least 1 device pass of assigned first-allocated endovascular strategy. Post hoc sensitivity analyses were conducted for reperfusion outcomes by excluding missing core laboratory data (complete cases analysis) and by handling missing core laboratory data with a multiple imputation procedure. Treatment effect modification on the primary outcome was explored in prespecified subgroups: age (≤70 vs >70 years), time from symptom onset to randomization (≤300 vs >300 minutes), occlusion site (isolated middle cerebral artery vs terminus internal carotid artery), intravenous thrombolysis (yes vs no), clot burden score (<6 vs ≥6), and collateral status (good vs poor) by including the corresponding interaction term into the multivariable mixed logistic-regression model. All statistical tests were 2-sided and a P value of less than .05 was considered statistically significant. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary outcomes and subgroup analyses should be considered as exploratory. Data were analyzed using the SAS software package, release 9.4 (SAS Institute).
Results
Randomization and Baseline Characteristics
Between October 16, 2017, and May 29, 2018, a total of 408 patients were enrolled from 11 centers (median number of patients per center, 30 [range, 3-120]; eTable 1 in Supplement 3). Participants were randomized into 2 groups: 205 patients to the experimental intervention group to undergo thrombectomy via initial contact aspiration and stent retriever combined and 203 patients to the control group for thrombectomy via initial stent retriever alone. Twenty-three patients from the experimental intervention group and 35 patients from the control group did not receive the allocated intervention, mainly due to spontaneous clot lysis observed in 7.8% (16) of patients the experimental intervention group and 6.9% (14) of patients the control group (Figure 1). Of the randomized patients, 2 patients from the experimental intervention group (1 because of recurrent inclusion in the trial and because of being under guardianship) and 1 patient from the control group (patient in whom deferred consent could not be obtained as the patient was not competent and no legally authorized representative was available to provide proxy consent) were excluded from analysis. Thus, 203 patients assigned to the combined contact aspiration and stent retriever group and 202 patients assigned to the stent retriever alone group were included in the primary efficacy analysis (Figure 1). As shown in Table 1, the baseline characteristics were well-balanced between the 2 study groups, with a mean (SD) age of 73.4 (13.9) years, 220 (54.3%) women, 185 (45.7%) men, and a mean baseline NIHSS score of 16.2 (6.3) (score range, 0 [no symptoms] to 42 [most severe neurological deficits]). Most patients (n = 345 [85.2%]) were included with an isolated middle cerebral artery occlusion at initial noninvasive imaging (Table 2).
Table 1. Baseline Patient Characteristics and Medical History.
| Characteristics | First-allocated thrombectomy groupa | |
|---|---|---|
| Contact aspiration and stent retriever combined (n = 203)b | Stent retriever alone (n = 202)b | |
| Age, mean (SD), y | 73.6 (14.3) | 73.3 (13.5) |
| Women | 104 (51.2) | 116 (57.4) |
| Men | 99 (48.8) | 86 (42.6) |
| Medical history | ||
| Hypertension | 124/200 (62.0) | 139/196 (70.9) |
| Previous antithrombotic medications | 98/200 (48.3) | 94/196 (46.5) |
| Antiplatelet | 55 (27.1) | 61 (30.2) |
| Anticoagulant | 50 (24.6) | 33 (16.3) |
| Previous atrial fibrillation | 72/197 (36.6) | 62/195 (31.8) |
| Hypercholesterolemia | 54/198 (27.3) | 71/192 (37.0) |
| Previous stroke or transient ischemic attack | 35/200 (17.5) | 29/197 (14.7) |
| Coronary artery disease | 34/197 (17.3) | 32/193 (16.6) |
| Diabetes | 34/200 (17.0) | 35/195 (18.0) |
| Current smoking | 18/155 (11.6) | 32/154 (20.8) |
Numeric values are reported as No. (%) or as No./total No. (%) unless otherwise indicated. Because medical history was based on medical record review, some data are missing.
Indicates the denominator, unless otherwise specified.
Table 2. Patients’ Stroke Characteristics at Baseline.
| Characteristics | First-allocated thrombectomy groupa | |
|---|---|---|
| Contact aspiration and stent retriever combined (n = 203)b | Stent retriever alone (n = 202)b | |
| Stroke event values and scores upon admission | ||
| Systolic blood pressure, mm Hg | 148 (25) [n = 191] | 150 (28) [n = 187] |
| Glucose, median (IQR), mmol/L | 6.9 (5.8 to 8.2) [n = 155] | 6.8 (5.8 to 8.0) [n = 162] |
| NIHSS score, mean (SD)c | 16.0 (6.2) [n = 201] | 16.3 (6.4) [n = 201] |
| ASPECT, (median IQR), scored | 7 (5 to 9) [n = 202] | 7 (6 to 9) [n = 201] |
| Site of occlusion before randomizationd,e | ||
| Isolated middle cerebral artery | 172 (84.7) | 173 (85.6) |
| Anterior cerebral artery | 31 (15.3) | 29 (14.4) |
| Angiographically determined occlusion sitef | ||
| M1-middle cerebral artery | 128/187 (68.4) | 111/186 (59.7) |
| Intracranial internal carotid artery | 30/187 (16.0) | 33/186 (17.7) |
| M2-middle cerebral artery | 22/187 (11.8) | 38/186 (20.4) |
| Tandem extracranial and intracranial occlusion | 4/187 (2.1) | 4/186 (2.2) |
| Extracranial internal carotid artery | 2/187 (1.1) | 0/186 |
| Anterior cerebral artery | 1/187 (0.5) | 0/186 |
| Clot burden, (median IQR), scoreg | 7 (5 to 8) [n = 143] | 7 (6 to 8) [n = 133] |
| Clot length, (median IQR), mmh | 11 (7 to 18) [n = 169] | 11 (7 to 15) [n = 161] |
| Favorable collateralsi | 55/169 (32.5) | 40/157 (25.5) |
| Suspected stroke cause | ||
| Cardioembolic | 103 (50.7) | 92 (46.0) |
| Other or unknown | 84 (41.4) | 94 (46.5) |
| Large artery atherosclerosis | 16 (7.9) | 15 (7.4) |
| Directly admitted to a comprehensive stroke centerj | 78 (38.4) | 70 (34.7) |
| Intravenous rt-PAk | 109 (53.7) | 110 (54.5) |
| Onset to intravenous rt-PA time, min | 150 (120 to 180) | 150 (123 to 195) |
| General anesthesia | 20/198 (10.1) | 19/198 (9.6) |
| Onset to arterial puncture time, min | 240 (185 to 306) | 246 (200 to 310) |
| Onset to imaging | 118 (88 to 160) | 119 (90 to 161) |
| Onset to randomization | 209 (172 to 283) | 229 (179 to 284) |
| Randomization to arterial puncture | 14 (2 to 30) | 11 (2 to 27) |
| Imaging to randomization | 83 (51 to 140) | 101 (58 to 146) |
| Imaging to room arrival | 95 (48 to 141) | 110 (48 to 150) |
| Room arrival to arterial puncture | 16 (12 to 26) | 16 (11 to 23) |
Abbreviations: ASPECT, Alberta Stroke Program Early CT (computed tomography); NIHSS, National Institutes of Health Stroke Scale; rt-PA, recombinant tissue plasminogen activator.
SI conversion factor: to convert glucose values to mg/dL, divide by 0.0555.
Numeric values are reported as No. (%) or as No./total No. (%) unless otherwise indicated. Because medical history was based on medical record review, some data are missing.
Indicates the denominator, unless otherwise specified.
Quantifies neurologic deficits in 11 categories (score range, 0 [no symptoms] to 42 [most severe neurological deficits]).
Measures the extension of stroke (score range, 0-10 [higher scores indicating fewer early ischemic changes]).
Site of occlusion was assessed at initial noninvasive imaging to determine the ASPECT score, which is imaging based.
Assessed angiographically by an independent core laboratory (not available for spontaneous clot revascularization [n = 30] and patients without arteriography [n = 2]).
Score indicates a semiquantitative CT angiography or magnetic resonance angiography–based scale, which assesses the number of arterial segments exhibiting a visible clot (range, 0-10 [higher clot burden translates as a lower clot burden score]).
Assessment was by an indepentent core laboratory after excluding patients with poor-quality images, incomplete examinations, or nondetected clot.
The collateral score range was from 0 (poor collateral supply) to 4 (good collateral supply) per the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology grading system. The favorable collateral score refers to grades 3 to 4.
Defined as a primary stroke center with availability of endovascular facilities and personnel trained in endovascular procedures.
Indicates patients treated by intravenous rt-PA within 4.5 hours from onset or based on diffusion-weighted imaging–positive or fluid-attenuated inversion recovery–negative mismatch among unknown stroke onset.
For the 366 patients who received at least 1 pass of the thrombectomy device, endovascular procedure and devices used are detailed according to study group (eTable 1 and eTable 2 in Supplement 3). Endovascular techniques were largely standardized, with a balloon-guide catheter employed to allow proximal flow arrest during stent retriever removal in 95.1% of patients from the experimental intervention group and 90.6% of patients from the control group (eTable 3 in Supplement 3).
Angiographic Efficacy Outcomes
The primary efficacy outcome (eTICI 2c/3 reperfusion after endovascular procedures) was not significantly different in the first-allocated thrombectomy contact aspiration and stent retriever combined group vs the stent retriever alone group (64.5% [n = 131/203] vs 57.9% [n = 117/202]; adjusted absolute risk difference, 6.6% [95% CI, –3.0% to 16.2%]; adjusted OR, 1.33 [95% CI, 0.88 to 1.99]; P = .17; Table 3). Regarding prespecified secondary outcomes, a significantly higher rate of eTICI 2b50/2c/3 and eTICI 2c/3 reperfusions after the assigned first-allocated intervention alone was observed in favor of the contact aspiration and stent retriever combined group (for eTICI 2b50/2c/3, 86.2% vs 72.3%; adjusted OR, 2.54 [95% CI, 1.51 to 4.28]; P < .001, and for eTICI 2c/3, 59.6% vs 49.5%; adjusted OR, 1.52 [95% CI, 1.02 to 2.27]; P = .04.
Table 3. Primary and Secondary Efficacy Outcomes.
| First-allocated thrombectomya | |||||
|---|---|---|---|---|---|
| Contact aspiration and stent retriever (n = 203) | Stent retriever (n = 202) | Adjusted absolute difference (95% CI) | Adjusted odds ratio (95% CI) | P value | |
| Primary efficacy outcome | |||||
| Reperfusion (eTICI 2c/3) at end of procedurea,b | 131 (64.5) | 117 (57.9) | 6.6 (–3.0 to 16.2) | 1.33 (0.88 to 1.99) | .17 |
| Secondary angiographic efficacy outcomes | |||||
| Reperfusion outcomes at end of procedureb | |||||
| eTICI 2b50/2c/3 | 183 (90.2) | 173 (85.6) | 5.0 (–1.6 to 11.1) | 1.62 (0.86 to 3.04) | .13 |
| eTICI 3 | 65 (32.0) | 56 (27.7) | 4.5 (–4.3 to 13.7) | 1.24 (0.80 to 1.92) | .32 |
| Reperfusion outcomes after the assigned initial intervention aloneb | |||||
| eTICI 2b50/2c/3 | 175 (86.2) | 146 (72.3) | 14.2 (5.8 to 21.8) | 2.54 (1.51 to 4.28) | <.001 |
| eTICI 2c/3 | 121 (59.6) | 100 (49.5) | 10.3 (0.8 to 20.1) | 1.52 (1.02 to 2.27) | .04 |
| eTICI 3 | 62 (30.5) | 50 (24.8) | 9.4 (–0.01 to 19.1) | 1.35 (0.86 to 2.11) | .18 |
| First-pass effect (eTICI 2c/3 after first pass) | 83 (40.9) | 68 (33.7) | 7.4 (–2.1 to 16.6) | 1.38 (0.91 to 2.08) | .12 |
| Modified first-pass effect (eTICI 2b50/2c/3 after first passc) | 109 (53.7) | 90 (44.6) | 9.4 (–0.01 to 19.1) | 1.48 (0.99 to 2.20) | .056 |
| eTICI 3 after first pass | 49 (24.1) | 36 (17.8) | 6.6 (–1.1 to 15.0) | 1.51 (0.92 to 2.48) | .10 |
| Use of rescue treatmentd | 39 (19.2) | 54 (26.7) | –7.6 (–14.8 to 0) | 0.61 (0.37 to 1.00) | .048 |
| Arterial puncture to eTICI 2c/3 reperfusion time, mine | |||||
| Median (IQR) | 42 (28 to 60) [n = 115] | 44 (29 to 64) [n = 103] | |||
| Mean (95% CI)f | 3.81 (3.67 to 3.94) | 3.80 (3.66 to 3.94) | 0.002 (–0.14 to 0.15)g | .98 | |
| Clot contact to maximal reperfusion time, minh | |||||
| Median (IQR) | 18 (9 to 44) [n = 182] | 22 (10 to 45) [n = 177] | |||
| Mean (95% CI) | 3.09 (2.89 to 3.29) | 3.18 (2.98 to 3.38) | –0.093 (–0.30 to 0.12)d | .37 | |
| Clinical efficacy outcomes | |||||
| Change in NIHSS score at 24 h, mean (95% CI)i | –3.1 (–4.4 to –1.7) [n = 193] | –2.7 (–4.0 to –1.3) [n = 196] | –0.4 (–2.3 to 1.5) | .67 | |
| Functional independence at 3 moj | 73/192 (38.0) | 80/191 (41.9) | –3.3 (–13.0 to 6.5) | 0.86 (0.56 to 1.33) | .50 |
| Functional independence at 12 moj | 74/173 (42.8) | 71/175 (40.6) | 3.5 (–6.7 to 13.3) | 1.17 (0.74 to 1.85) | .49 |
| EQ-5D utility index score at 12 mok | |||||
| Median (IQR) | 0 (0 to 0.84) [n = 181] | 0.27 (0 to 0.80) [n = 178] | |||
| Mean (95% CI) | 0.34 (0.28 to 0.44) | 0.36 (0.28 to 0.41) | –0.02 (–0.11 to 0.07) | .59 | |
| EQ-5D VAS at 12 mo, mean (95% CI)l | 69 (63 to 75) [n = 95] | 63 (57 to 69) [n = 103] | 6.00 (–0.03 to 12.22) | .051 | |
Abbreviations: EQ-5D, European Quality of Life 5-Dimension; eTICI, expanded Treatment in Cerebral Infarction score; NIHSS, National Institutes of Health Stroke Scale; VAS, visual analog scale.
eTICI score explanation: a 0 to 3 visual scale scoring cerebral perfusion on digital subtraction angiography. A score of 2c means near-total cerebral perfusion except for slow flow in a few distal cortical vessels or presence of small distal cortical emboli; a score of eTICI 3 means total cerebral perfusion with normal filling of all distal branches.
eTICI was not assessed by the core laboratory. At both the end of the endovascular procedure and at initial strategy, eTICI was imputed as the following: (1) failure due to no device pass (6 for intracranial access failure [1 in the combined contact aspiration and stent retriever group and 5 in stent retriever group] and 3 decisions of the physician to not perform thrombectomy [1 in the combined contact aspiration and stent retriever group and 2 in stent retriever group]); and (2) success (as any eTICI grades) due to spontaneous recanalization (n = 30 [16 in the combined contact aspiration and stent retriever group and 14 in stent retriever group]). Imputation values differed for eTICI graded by the treating physician due to unavailable or poor-quality images (for the end of the endovascular procedure, n = 12 [5 in the combined contact aspiration and stent retriever group and 7 in stent retriever group]; for the initial strategy, n = 6, all in stent retriever group) (eMethods in Supplement 3).
Indicates eTICI 2b50/2c/3 after first pass without adjuvant therapy.
In post-hoc analysis, this indicates a switch of mechanical thrombectomy strategy following failure to achieve eTICI 2c/3 after a maximum of 3 passes of the initial assigned mechanical thrombectomy strategy.
Data were available for patients with eTICI 2c/3 reperfusion after at least 1 device pass.
Indicates difference in mean values.
Calculated after log-transformation of values.
Data among patients with at least 1 device pass.
Adjusted for baseline NIHSS score.
Indicates modified Rankin scale score less than or equal to 2 (measures the degree of disability; score range, 0 [symptom free] to 6 [dead]).
Indicates a descriptive profile and single index value for health status—a self-reported and preference-based quality-of-life measure with 1 question for each of the 5 dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression). The answers describe 243 unique health states or can be converted into a utility score (range, 0 [death] to 1 [perfect health]).
Records self-rated health status on a graduated scale (0-100 [higher scores for higher health-related quality of life]); indicates patients alive at 12 months. Effect sizes (adjusted absolute difference and adjusted odds ratio) were calculated after adjustment for randomization stratification variables (center, age [<70 vs ≥70 years], intravenous thrombolysis and occlusion site [isolated middle cerebral artery vs tandem middle cerebral artery and internal cerebral artery]).
In addition, prespecified angiographic outcomes after first pass alone demonstrated a 40.9% eTICI 2c/3 reperfusion for the contact aspiration and stent retriever combined group vs 33.7% eTICI 2c/3 reperfusion for the stent retriever alone group (OR, 1.38 [95% CI, 0.91 to 2.08]; P = .12). Likewise, prespecified angiographic outcomes after first pass demonstrated eTICI 2b50/2c/3 reperfusion in 53.7% of patients in the contact aspiration and stent retriever combined group vs 44.6% of patients in the stent retriever alone group (OR, 1.48 [95% CI, 0.99 to 2.20]; P = .06). A significantly lower rate of rescue treatment was also observed after combined contact aspiration and stent retriever thrombectomy vs stent retriever alone (19.2% vs 26.7%; adjusted OR, 0.61 [95% CI, 0.37 to 1.00]; P = .05, post hoc analysis) (eTable 4 in Supplement 3).
Among patients reaching eTICI 2c/3 reperfusion after at least 1 device pass, the median time from arterial puncture to reperfusion was not significantly different between groups (42 minutes [IQR, 28 to 60] in the contact aspiration and stent retriever combined group vs 44 minutes [IQR, 29 to 64] in the stent retriever alone group). Similarly, there were no significant differences in time between clot contact and maximal reperfusion (Table 3).
Similar results on angiographic outcomes were found in prespecified per-protocol analysis (eTable 5 in Supplement 3), as well as in post hoc complete case analysis (excluding missing values in eTICI scores [eTable 6 in Supplement 3]) and multiple imputation analysis (handling missing eTICI scores using 10 imputations whatever the reason for missing values [eTable 7 in Supplement 3]). In the prespecified sensitivity analysis using primary outcome assessed by study site (eTICI graded by the treating physician), primary outcome was achieved in 62.6% [n = 127] of patients in the contact aspiration and stent retriever combined group and in 57.9% [n = 117] of patients in the stent retriever alone group (adjusted OR, 1.24 [95% CI, 0.82 to 1.88]; P = .30).
We assessed the reproducibility of core laboratory readers to grade eTICI (by pooling initial, first-line and end of procedure angiography imaging) and found an excellent agreement with a weighted κ coefficient of 0.91 (95% CI, 0.89 to 0.94) and a concordance rate of 86.3%.
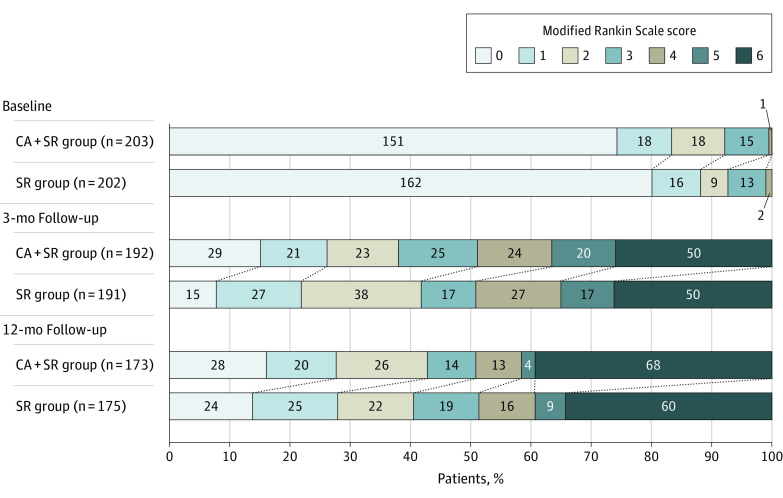
Clinical Efficacy Outcomes
There was no significant difference in NIHSS score change from admission to 24 hours, and there was no significant difference in the degree of disability or functional independence at 3- and 12-month follow-up between the 2 study groups in primary analysis (Figure 2 and Table 3) or per-protocol analysis (eTable 5 in Supplement 3). Regarding the health-related quality of life assessed among patients at 12-month follow-up, no significant differences were found in the quality-of-life score (assessed using the EQ-5D visual analog scale) in patients treated by first-allocated combined contact aspiration and stent retriever thrombectomy compared with stent retriever alone, with a mean difference of 6.0 (69 vs 63 [95% CI, –0.03 to 12.22]; P = .05) in the main analysis and 5.9 (70 vs 64; OR, 5.88 [95% CI, –0.91 to 12.68]; P = .09 in the per-protocol analysis.
Figure 2. Distribution of the Modified Rankin Scale Score at Baseline (prestroke), 3 Months, and 12 Months.
The modified Rankin Scale score measures the degree of disability (score range, 0 [symptom free] to 6 [dead]). The common adjusted odds ratio for a 1-point improvement in the modified Rankin Scale score associated with the combined contact aspiration and stent retriever group was 0.93 (95% CI, 0.64 to 1.34; P = .68) at 3 months and 0.91 (95% CI, 0.61 to 1.35; P = .65) at 12 months. CA indicates contact aspiration; SR, stent retriever.
Safety Outcomes
There were no significant differences in safety outcomes (Table 4), except for a significantly lower rate of type 2 parenchymal hematoma in the contact aspiration and stent retriever combined group (3.1%) compared with the stent retriever alone group (8.6%; adjusted OR, 0.34 [95% CI, 0.12 to 0.88]). Procedure-related adverse events occurred in 51 patients (25.1%) after first-allocated combined contact aspiration and stent retriever thrombectomy and 49 patients (24.3%) after stent retriever alone. The most frequent event was vasospasm (n = 39), followed by embolization in a new territory (n = 23), arterial dissection (n = 7), and arterial perforation (n = 2). During the 12-month follow-up, 127 patients died with a 12-month cumulative incidence of 35.8% in the contact aspiration and stent retriever combined group vs 31.1% in the stent retriever alone group (eFigure 1 and eFigure 4 in Supplement 3).
Table 4. Adverse Events.
| No./total No. (%) for first-allocated thrombectomy | ||
|---|---|---|
| Contact aspiration and stent retriever combined (n = 203) | Stent retriever alone (n = 202) | |
| Procedure-related adverse eventsa,b | 51 (25) | 49 (24) |
| Spasm | 23/203 (11) | 16/202 (8) |
| Embolization in a new territory | 12 (6) | 11/202 (5) |
| Arterial dissection | 1/203 (0.5) | 6/202 (3) |
| Arterial perforation | 0/203 (0) | 2/202 (1) |
| New ischemic stroke in a different vascular territory within 24 h of procedure | 33/194 (17) | 39/198 (20) |
| Intracranial hemorrhage at 24 ha,b | 94/195 (48) | 98/197 (50) |
| Hemorrhagic infarctionc | 69/195 (35) | 57/197 (29) |
| Type 2 | 45/195 (23) | 33/197 (17) |
| Type 1 | 24/195 (12) | 24/197 (12) |
| Subarachnoid hemorrhage | 21/195 (11) | 21/197 (11) |
| Parenchymal hematomad | 19/195 (10) | 32/197 (16) |
| Type 1 | 13/195 (7) | 15/197 (8) |
| Type 2 | 6/195 (3) | 17/197 (9) |
| Intraventricular hemorrhage | 7/195 (4) | 12/197 (6) |
| Remote intracranial hemorrhage | 1/195 (0.5) | 3/197 (2) |
| Symptomatic intracranial hemorrhagee at 24 h | 13/195 (7) | 16/197 (8) |
| All-cause mortality at 3 mo | 50/195 (26) | 50/197 (25) |
| All-cause mortality at 12 mo | 67/174 (39) | 60/175 (34) |
Assessed by the core laboratory.
Multiple events of 1 type were counted once
Type 1 indicates scattered small petechia with no mass effect; type 2, confluent petechia with no mass effect.
Type 1 indicates hematoma within infarcted tissue occupying less than 30% with no substantive mass effect; type 2, hematoma occupying 30% or more of the infarcted tissue with obvious mass effect.
Defined according to the European Cooperative Acute Stroke Study III classification (+4 points in the NIHSS between baseline and day 1 with a causal relationship).14
Subgroup Analyses
In prespecified exploratory subgroup analyses, we found no heterogeneity in treatment effect on the primary outcome.(eFigure 2 in Supplement 3).
Discussion
This randomized trial did not demonstrate any significant increase in near-total or total reperfusion (eTICI 2c/3) at the end of the endovascular procedure with the combined initial contact aspiration and stent retriever technique compared with stent retriever technique alone among patients presenting anterior circulation large vessel occlusion strokes within 8 hours of symptoms onset.
The eTICI 2c/3 reperfusion grade at the end of the endovascular procedure was chosen as the primary outcome as it represents a strong early indicator of treatment success and has been correlated with a better clinical outcome with lower disability when compared with patients with eTICI 2b.4,18,22 Thus, achieving an eTICI 2c/3 reperfusion is now the generally accepted goal of mechanical thrombectomy procedures. The overall rate of eTICI 2c/3 and 2b50/2c/3 reperfusion observed here for the stent retriever thrombectomy group was higher than those reported in the other major randomized trials, which demonstrated the superiority of mechanical thrombectomy over medical treatment alone (57.9% and 85.6% vs 32% and 71% from the HERMES Collaborative Group,4 (eTable 3 in Supplement 3). This may be explained by the mandatory use (more than 90% in both groups) of the balloon-guide catheter, as already suggested.23,24 The high rate of eTICI 2b50/2c/3 and eTICI 2c/3 after stent retriever thrombectomy alone could explain the absence of detection of superiority of combined contact aspiration and stent retriever compared with stent retriever alone observed in this trial.
For the primary outcome, the 95% CI for the between-group difference extends up to 16.2% and thus includes the 15% difference that the study was powered to detect. The study may have been underpowered to detect smaller benefits in favor of the combined approach with regard to the primary outcome.
The direct measure of the efficacy of the first-allocated endovascular procedure assessed by the rate of eTICI 2c/3 or eTICI 2b50/3 at the end of the first strategy, ie, contact aspiration and stent retriever combined vs stent retriever alone thrombectomy, showed an effect of combined contact aspiration and stent retriever vs stent retriever alone among prespecified angiographic outcomes. Combined contact aspiration and stent retriever mechanical thrombectomy yielded significantly better results than stent retriever alone in terms of eTICI 2c/3 and eTICI 2b50/2c/3 (Table 3).
Therefore, these data may support the increased effectiveness of initial combined endovascular strategies compared to stent retriever alone as already reported among observational studies.10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25
This result was also confirmed by the fact that more additional rescue therapies were necessary in the stent retriever alone group (post hoc analysis). Nevertheless, the procedure times did not differ significantly. This apparent discrepancy is explained by the fact that in the stent retriever alone group, the second-line strategy mainly consisted of adding an aspiration catheter either as a stand-alone device or in conjunction with the stent retriever. The time initially gained by not navigating an aspiration catheter in the stent retriever group was subsequently lost by doing so for the second-line strategy, thus resulting in similar total procedure times between the stent retriever alone group and the stent retriever and contact aspiration combined group. These findings suggested that contact aspiration and stent retriever combined may be more efficient to obtain successful recanalization but that this effect disappeared after rescue therapy.
However, eTICI 2b50/3 and eTICI 2c/3 reperfusion rates with the first-allocated strategy were significantly higher in the combined group, and point estimates for the other angiographic outcomes also favored the combined approach, though did not reach statistical significance (shown in Table 3).
Additionally, the core laboratory–adjudicated safety outcomes between the 2 groups did not suggest any significant difference in terms of symptomatic ICH or embolization in a new territory (as previously reported10), and there was no significant difference in terms of mortality at 90 days.
Limitations
This study has several limitations. First, the sample size was based on the assumption of a rate of eTICI 2c/3 in the control group equal to 55%. A similar value (57.9%) was observed in the stent retriever group. Unfortunately, a treatment effect of 15% was expected, which was above that observed in the experimental intervention group. This expected rate in the combined group was based on available data at the time of trial set-up (2016) and not on a minimal clinically important difference, which has been evaluated between 3.1% to 5%.26 The trial was underpowered to detect smaller between-group differences, and more trials are needed for a more precise effect estimate.
Second, the external validity of the angiographic results is limited given that participating centers usually performed more than 150 procedures per year. Moreover, certain physicians will always have their preferred technique and feel more comfortable with one approach vs another. The skillset and experience of individuals will also vary, which introduces clinician-based inconsistency in technique.
Third, mechanical thrombectomy techniques are rapidly evolving, and as such, the results of this trial, although valid at present, may be different if the trial is repeated in a few years’ time. For example, with very large–bore distal aspiration catheters becoming available, aspiration may become more effective. At the same time, there are now attempts to design stent retrievers with a geometry that is specifically tailored toward certain histological thrombus compositions. These results are subject to change with the evolution of mechanical thrombectomy technology.
Fourth, the mRS outcome scores were determined in a patient/proxy-reported outcome manner by informants not blinded to treatment assignment.
Conclusions
Among patients with acute ischemic stroke due to large vessel occlusion, an initial thrombectomy technique consisting of contact aspiration and stent retriever combined, compared with stent retriever alone, did not significantly improve the rate of near-total or total reperfusion (eTICI 2c/3) at the end of the endovascular procedure, although the trial may have been underpowered to detect smaller differences between groups.
Trial Protocol
Statistical Analysis Plan
eAppendix. Definitions of eTICI and mRankin Score
eMethods. Interventions and Thrombectomy Procedure
eTable 1. Number of Patients Included Per Participating Center
eTable 2. Procedural Details (in Analyzed Population After at Least 1 Device Pass)
eTable 3. Detail of Thrombectomy Devices Used in Frontline and Rescue Strategies According to the Assigned Groups
eTable 4. Data on Angiographic and Clinical Outcomes on the Rescue Therapy Group
eTable 5. Primary and Secondary Efficacy Outcomes in Per-Protocol Population
eTable 6. Primary and Secondary Reperfusion Outcomes in Complete Case Analysis
eTable 7. Primary and Secondary Reperfusion Outcomes in Multiple-Imputation Analysis
eFigure 1. Cumulative Incidence of All-Cause Mortality During the 12-Month Follow-up Period
eFigure 2. Treatment Effect Size (First-Line CA/SR Thrombectomy vs First-Line SR Alone Thrombectomy) on Primary Outcome According to Key Subgroups
eFigure 3. Conditional Studentized Residuals of Linear Mixed Models (Including the Constrained Longitudinal Data Analysis) for Secondary Quantitative Outcomes
eFigure 4. Schoenfeld Residuals Plot for Treatment Group Derived From Cox Regression Model of 12-Month All-Cause Mortality
Nonauthor Collaborator List
Data Sharing Statement
References
- 1.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 2.Turk AS III, Siddiqui A, Fifi JT, et al. Aspiration thrombectomy versus stent retriever thrombectomy as first-line approach for large vessel occlusion (COMPASS): a multicentre, randomised, open label, blinded outcome, non-inferiority trial. Lancet. 2019;393(10175):998-1008. doi: 10.1016/S0140-6736(19)30297-1 [DOI] [PubMed] [Google Scholar]
- 3.Lapergue B, Blanc R, Gory B, et al. ; ASTER Trial Investigators . Effect of endovascular contact aspiration vs stent retriever on revascularization in patients with acute ischemic stroke and large vessel occlusion: the ASTER randomized clinical trial. JAMA. 2017;318(5):443-452. doi: 10.1001/jama.2017.9644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liebeskind DS, Bracard S, Guillemin F, et al. ; HERMES Collaborators . eTICI reperfusion: defining success in endovascular stroke therapy. J Neurointerv Surg. 2019;11(5):433-438. doi: 10.1136/neurintsurg-2018-014127 [DOI] [PubMed] [Google Scholar]
- 5.Powers WJ, Rabinstein AA, Ackerson T, et al. ; American Heart Association Stroke Council . 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 6.Turc G, Bhogal P, Fischer U, et al. European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. doi: 10.1177/2396987319832140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgado Almandoz JE, Kayan Y, Young ML, et al. Comparison of clinical outcomes in patients with acute ischemic strokes treated with mechanical thrombectomy using either Solumbra or ADAPT techniques. J Neurointerv Surg. 2016;8(11):1123-1128. doi: 10.1136/neurintsurg-2015-012122 [DOI] [PubMed] [Google Scholar]
- 8.Jindal G, Serulle Y, Miller T, et al. Stent retrieval thrombectomy in acute stoke is facilitated by the concurrent use of intracranial aspiration catheters. J Neurointerv Surg. 2017;9(10):944-947. doi: 10.1136/neurintsurg-2016-012581 [DOI] [PubMed] [Google Scholar]
- 9.Massari F, Henninger N, Lozano JD, et al. ARTS (aspiration-retriever technique for stroke): initial clinical experience. Interv Neuroradiol. 2016;22(3):325-332. doi: 10.1177/1591019916632369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McTaggart RA, Tung EL, Yaghi S, et al. Continuous aspiration prior to intracranial vascular embolectomy (CAPTIVE): a technique which improves outcomes. J Neurointerv Surg. 2017;9(12):1154-1159. doi: 10.1136/neurintsurg-2016-012838 [DOI] [PubMed] [Google Scholar]
- 11.Lapergue B, Labreuche J, Blanc R, et al. Combined use of contact aspiration and the stent retriever technique versus stent retriever alone for recanalization in acute cerebral infarction: the randomized ASTER 2 study protocol. J Neurointerv Surg. 2020;12(5):471-476. doi: 10.1136/neurintsurg-2019-014735 [DOI] [PubMed] [Google Scholar]
- 12.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. [PubMed] [Google Scholar]
- 13.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 14.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee . Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25(5):457-507. doi: 10.1159/000131083 [DOI] [PubMed] [Google Scholar]
- 15.Humphries W, Hoit D, Doss VT, et al. Distal aspiration with retrievable stent assisted thrombectomy for the treatment of acute ischemic stroke. J Neurointerv Surg. 2015;7(2):90-94. doi: 10.1136/neurintsurg-2013-010986 [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 17.EuroQol Group . EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. doi: 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 18.Dargazanli C, Fahed R, Blanc R, et al. ; ASTER Trial Investigators . Modified thrombolysis in cerebral infarction 2C/thrombolysis in cerebral infarction 3 reperfusion should be the aim of mechanical thrombectomy: insights from the ASTER Trial (contact aspiration versus stent retriever for successful revascularization). Stroke. 2018;49(5):1189-1196. doi: 10.1161/STROKEAHA.118.020700 [DOI] [PubMed] [Google Scholar]
- 19.Goyal M, Fargen KM, Turk AS, et al. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg. 2014;6(2):83-86. doi: 10.1136/neurintsurg-2013-010665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno A, Shah N, Lin C, et al. Improving modified Rankin Scale assessment with a simplified questionnaire. Stroke. 2010;41(5):1048-1050. doi: 10.1161/STROKEAHA.109.571562 [DOI] [PubMed] [Google Scholar]
- 21.Austin PC. Absolute risk reductions, relative risks, relative risk reductions, and numbers needed to treat can be obtained from a logistic regression model. J Clin Epidemiol. 2010;63(1):2-6. doi: 10.1016/j.jclinepi.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 22.Kaesmacher J, Maegerlein C, Zibold F, Wunderlich S, Zimmer C, Friedrich B. Improving mTICI2b reperfusion to mTICI2c/3 reperfusions: a retrospective observational study assessing technical feasibility, safety and clinical efficacy. Eur Radiol. 2018;28(1):274-282. doi: 10.1007/s00330-017-4928-3 [DOI] [PubMed] [Google Scholar]
- 23.Nguyen TN, Malisch T, Castonguay AC, et al. Balloon guide catheter improves revascularization and clinical outcomes with the Solitaire device: analysis of the North American Solitaire Acute Stroke Registry. Stroke. 2014;45(1):141-145. doi: 10.1161/STROKEAHA.113.002407 [DOI] [PubMed] [Google Scholar]
- 24.Zaidat OO, Mueller-Kronast NH, Hassan AE, et al. ; STRATIS Investigators . Impact of balloon guide catheter use on clinical and angiographic outcomes in the STRATIS stroke thrombectomy registry. Stroke. 2019;50(3):697-704. doi: 10.1161/STROKEAHA.118.021126 [DOI] [PubMed] [Google Scholar]
- 25.Di Maria F, Kyheng M, Consoli A, et al. Identifying the predictors of first-pass effect and its influence on clinical outcome in the setting of endovascular thrombectomy for acute ischemic stroke: results from a multicentric prospective registry. Int J Stroke. 2021;16(1):20-28. doi: 10.1177/1747493020923051 [DOI] [PubMed] [Google Scholar]
- 26.Lin CJ, Saver JL. Noninferiority margins in trials of thrombectomy devices for acute ischemic stroke: is the bar being set too low? Stroke. 2019;50(12):3519-3526. doi: 10.1161/STROKEAHA.119.026717 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eAppendix. Definitions of eTICI and mRankin Score
eMethods. Interventions and Thrombectomy Procedure
eTable 1. Number of Patients Included Per Participating Center
eTable 2. Procedural Details (in Analyzed Population After at Least 1 Device Pass)
eTable 3. Detail of Thrombectomy Devices Used in Frontline and Rescue Strategies According to the Assigned Groups
eTable 4. Data on Angiographic and Clinical Outcomes on the Rescue Therapy Group
eTable 5. Primary and Secondary Efficacy Outcomes in Per-Protocol Population
eTable 6. Primary and Secondary Reperfusion Outcomes in Complete Case Analysis
eTable 7. Primary and Secondary Reperfusion Outcomes in Multiple-Imputation Analysis
eFigure 1. Cumulative Incidence of All-Cause Mortality During the 12-Month Follow-up Period
eFigure 2. Treatment Effect Size (First-Line CA/SR Thrombectomy vs First-Line SR Alone Thrombectomy) on Primary Outcome According to Key Subgroups
eFigure 3. Conditional Studentized Residuals of Linear Mixed Models (Including the Constrained Longitudinal Data Analysis) for Secondary Quantitative Outcomes
eFigure 4. Schoenfeld Residuals Plot for Treatment Group Derived From Cox Regression Model of 12-Month All-Cause Mortality
Nonauthor Collaborator List
Data Sharing Statement