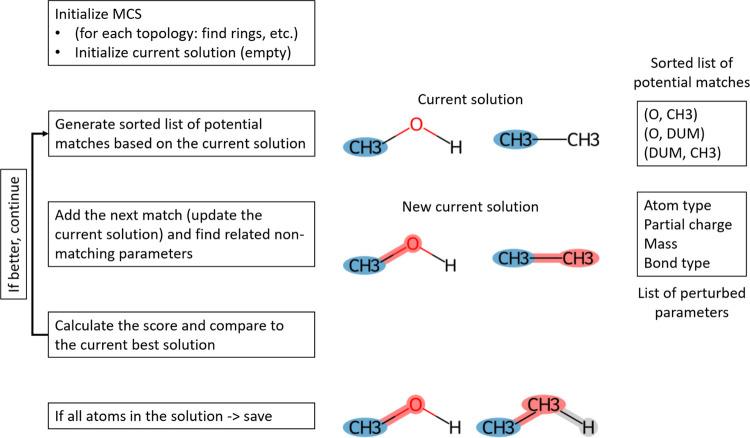
Figure 1.
General workflow of the perturbation topology builder. Algorithm steps are shown on the left side. Step (0) initializes all relevant attributes and parameters needed for the search and performs a simple analysis of the input topologies (e.g., finding rings). After the initialization step, an iterative search procedure is initiated. Step (1) based on the current solution (in the very first call, empty solution, i.e., no matched atoms) generates a list of all possible and allowed atom matches (pairs) and sorts them according to the estimated number of additional atom matches that are possible after the atom match in the question is added to the solution (related to the score calculated in step 3). Step (2) adds the next atom pair (match) from the sorted list from step 1 to the current solution and updates it. Step (3) calculates a score based on the user-defined function and compares it to the best score (the best solution) enumerated thus far in the execution of the algorithm. If the score is better than the one of the current best, the algorithm continues with a next iteration of step 1. If all atoms of all input topologies are present in the solution, the solution is saved. An illustration of the execution of the algorithm is shown on the right based on a toy perturbation problem between methanol and ethane (compounds represented according to the GROMOS force field where the methyl groups are modeled as a single united-atom particle). The current solution in step 1 with two methyl groups matched (in blue) is shown. Accordingly, the list of potential next atom matches is generated based on the first neighbors of the atoms in the current solution. Note that matches with dummy atoms are also explicitly included in the algorithm. The updated current solution after adding the next atom match between the oxygen and the second methyl in step 2 is shown (highlighted in red to notify perturbation in atom type, partial charge, and mass, with perturbed bonds also marked in red). Finally, the solution with the maximum number of matched atoms is shown at the bottom, where the hydrogen atom in the methanol state is marked for removal, i.e., perturbation into a noninteracting dummy atom and the other way around in the ethane state (dummy atom highlighted in light gray).