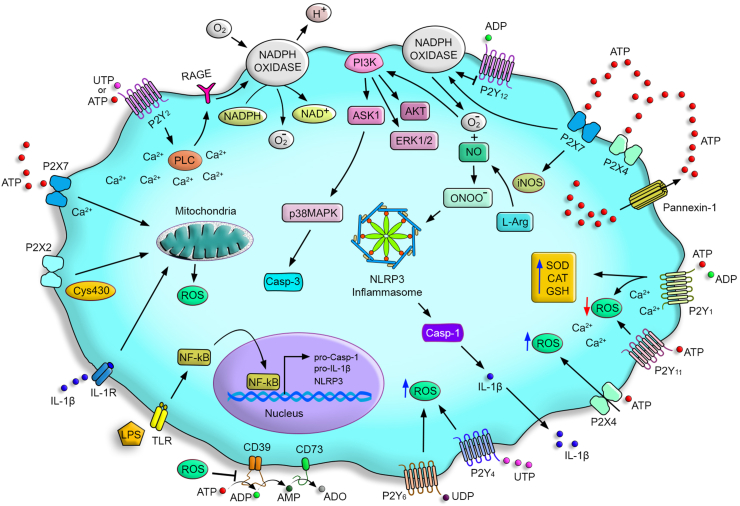
Fig. 2.
P2 receptors signaling pathways in the modulation of redox status. Pathogens can release pathogen-associated molecular patterns), such as lipopolysaccharides (LPS), which can activate pattern recognition receptors (PRRs) (i.g, TLRs). This activation leads to the transcription of genes involved in NLRP3 inflammasome activation (NLRP3, pro-casp 1, and pro-IL1β) and ATP release. ATP in high concentrations activates the P2X7 receptor inducing ROS production via NADPH oxidase activation and peroxynitrite. ROS is responsible for activating the phosphatidylinositol-3 kinase (PI3K) pathway, ASK1, AKT, ERK1/2, and p38 MAPK. These mechanisms can induce apoptosis via caspase-3 activation and NLRP3 inflammasome assembly, leading to caspase-1 activation and pro-IL-1β processing. Low concentrations of ATP or UTP can activate the P2Y2 receptor inducing PLC activation and Ca+2 mobilization; these components activate NADPH oxidase, responsible for producing ROS in a mechanism dependent on RAGE expression. The ATP also activates the P2X4 receptor that promotes ROS production and potentiates the activity of the P2X7 receptor for activating NADPH oxidase. The P2X7 also induces GSH release, increasing intracellular ROS. P2Y1 receptor increases the expression of antioxidant genes and activity of SOD, CAT, and GSH via intracellular Ca+2 signals. The P2Y1 receptor also induces the phosphorylation of endothelial nitric oxide synthase (eNOS). The sensor Cys430 allows the P2X2 receptor to sense the cell redox state in front of ROS production. P2Y4 and P2Y6 receptors promote ROS production, while the activation of the P2Y11 and P2Y12 receptor activation can inhibit ROS generation. ROS inhibits CD39 and CD73 activities.