Figure 1.
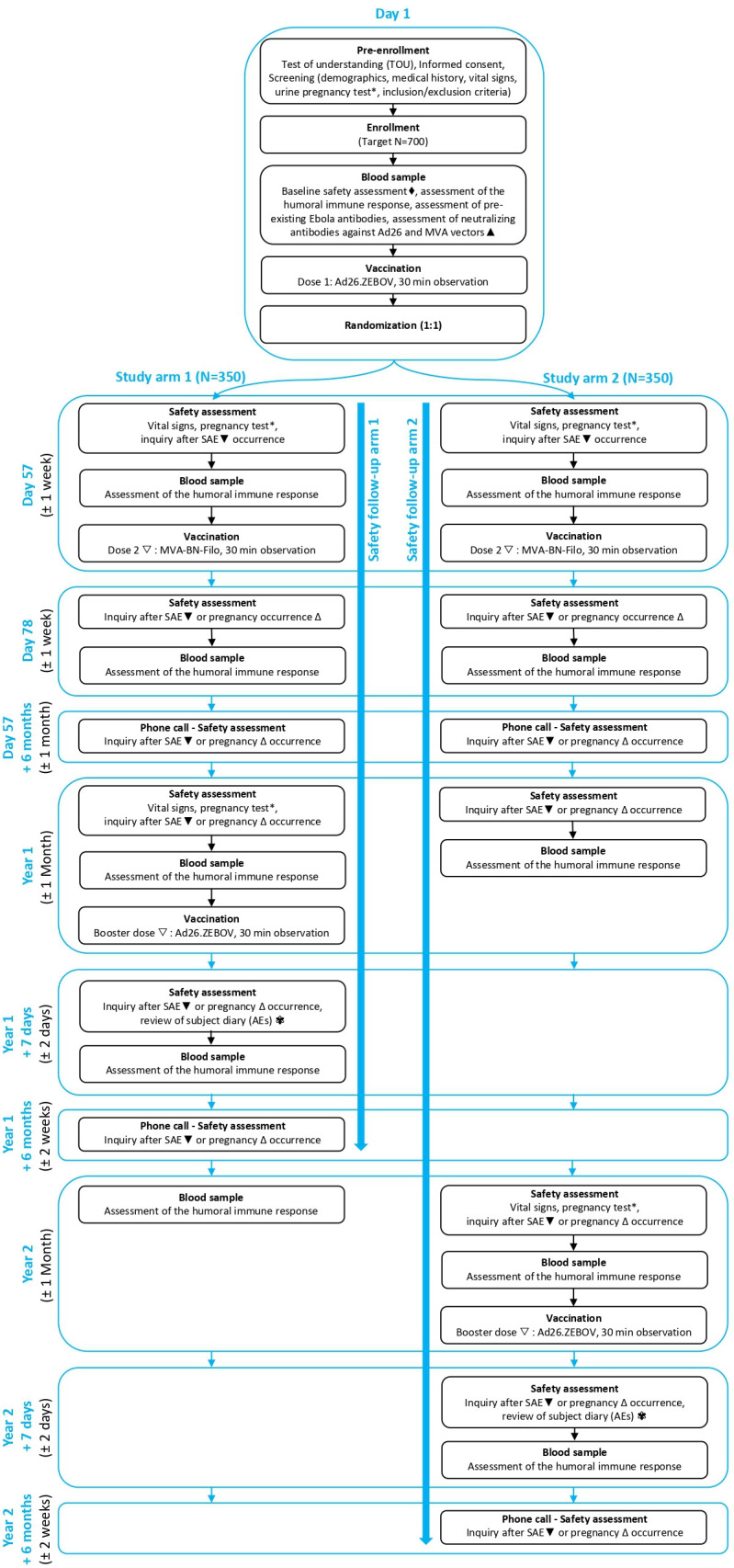
Study time and events overview. * Only for female participants of childbearing potential; ♦ Abnormal results will not exclude a participant, as results will not be reviewed prior to enrolment; ▲ Only the first 100 participants enrolled will be tested for neutralising antibody response against Ad26 virus neutralising assay and MVA vectors. Other blood analyses are for all 700 participants; ▼ Concomitant therapies given in conjunction with a serious adverse event (SAE) should be recorded from signing of the Informed Consent Form onwards until 6 months post booster; ▽ The investigator may withhold the second vaccine or booster dose if a participant’s clinical status changes prior to vaccination. The participant should continue to be followed for safety and immunogenicity according to the protocol; ∆ Only for female participants; ✾ Solicited and unsolicited adverse events (AEs) will be collected in a participant diary during 1 week post booster vaccination.
