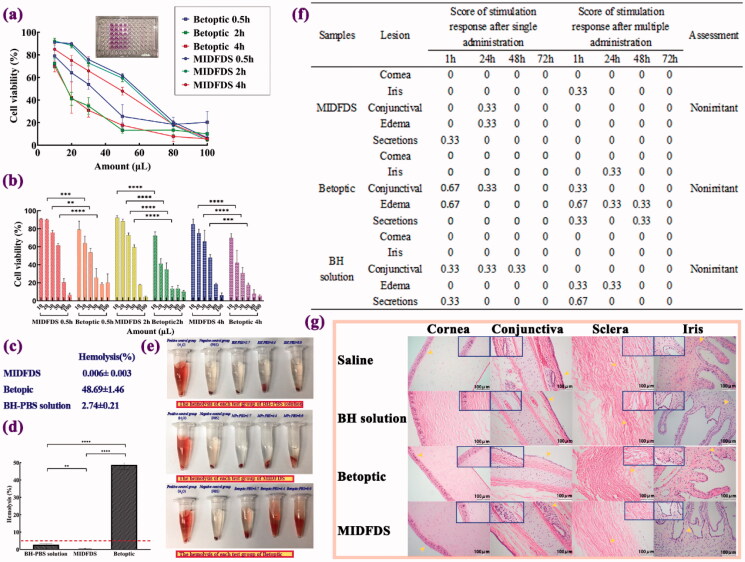
Figure 6.
Biocompatibility evaluation of Betoptic and MIDFDS. (a,b) Percent cell viability of each formulation after different exposure times and administration dosages (n = 3, mean ± SD). (c,d) Hemolysis percentage of the BH-PBS, Betoptic, and MIDFDS. The dashed line in (d) indicates 5% hemolysis. (e) The hemolysis of each test group after 4 h of incubation with erythrocytes. (f) Ocular irritation scores from rabbits (single and multiple administrations, n = 3). (g) Ocular tissues were stained with hemoxylin and eosin (H&E) following chronic application in the saline group, BH solution group, Betoptic group, and MIDFDS group (magnification 200×).