Figure 2. De novo purine synthesis is increased by oncogene activation, promotes pro-tumor cellular activities, and represents a therapeutic liability in HGG.
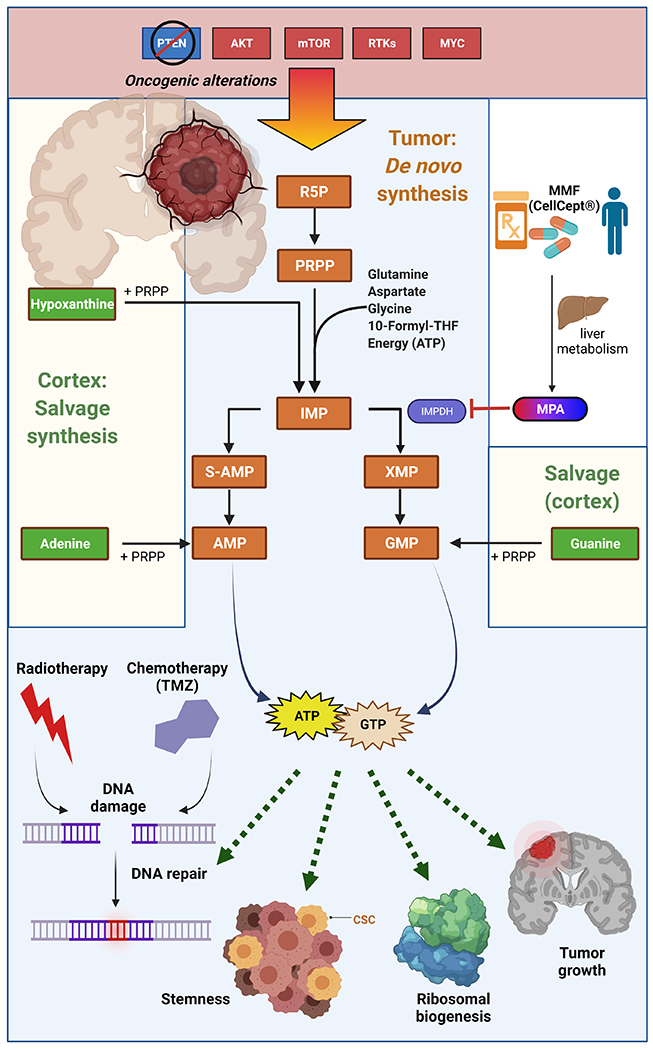
De novo purine synthesis is activated by oncogenic alterations including PTEN deletions and upregulation or mutations in AKT, mTOR, receptor tyrosine kinases (RTKs), and MYC. In the de novo purine synthetic pathway, ribose 5-phosphate (R5P) is activated to phosphoribosyl pyrophosphate (PRPP), allowing the biochemical construction of a purine ring upon the ribose unit to form inosine monophosphate (IMP). IMP can be converted to adenylosuccinate (S-AMP) and then adenosine monophosphate (AMP) and adenosine triphosphate (ATP). IMP can also be converted to xanthosine monophosphate (XMP) via IMP dehydrogenase (IMPDH) and then guanosine monophosphate (GMP) and guanosine triphosphate (GTP). In non-malignant brain tissue, purines are produced through salvage synthesis, in which free nitrogenous bases are conjugated to PRPP. Increased purine levels in HGGs promote stemness, tumor growth, ribosomal biogenesis, and DNA repair and subsequent therapeutic resistance. Purine synthesis can be pharmacologically targeted using mycophenolate mofetil (MMF/CellCept®), which is converted to mycophenolic acid (MPA) in the liver. MPA inhibits IMPDH to suppress purine synthesis and gliomagenesis in preclinical models. Figure created with BioRender.
