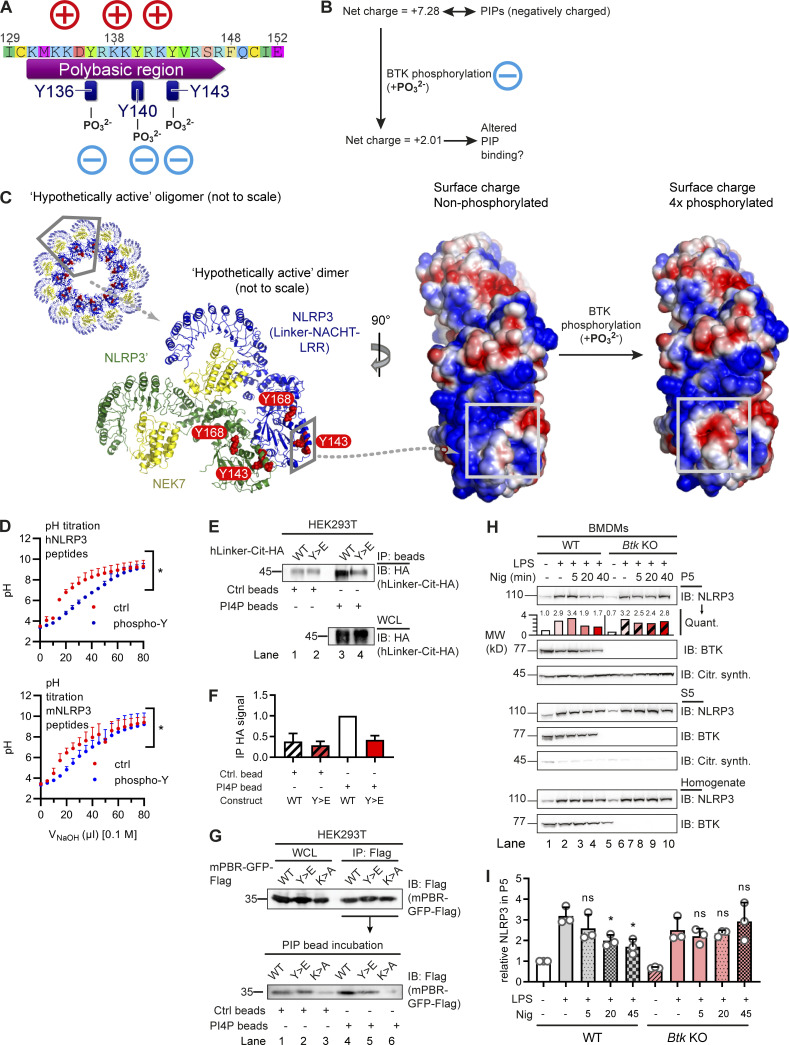
Figure 3.
BTK phosphorylation of the NLRP3 polybasic motif enables Golgi/PI4P dissociation. (A and B) Charge distribution (A) and ProtPi net charge computation (B) of unmodified and 3× phosphopeptide human NLRP3 PBR. (C) CHARMM surface charge predictions of linker–NACHT–LRR structure in the putative nonphosphorylated (left) and 4× phosphorylated (right) form. Blue, positive charge; red, negative charge. Gray boxes indicate that the area of charge alterations in the monomers maps to a contact area in the hypothetical dimer (center, rotated by 90°; see relative position in oligomer below). (D) pH titration of peptides encompassing the polybasic motifs of human or murine NLRP3 as phospho (blue) or non-phosphorylated control (ctrl; red) peptide (n = 3). (E and F) Human NLRP3 linker-Cit-HA constructs precipitated with PI4P beads (n = 2; quantified in F relative to immunoprecipitation [IP] HA signal in WT transfection [lane 1]). (G) As in E but murine NLRP3 PBR fused to GFP-Flag (mPBR-GFP-Flag; n = 2). (H and I) Subcellular fractionation of nigericin-treated WT or Btk KO BMDM lysates into P5 (heavy membranes) and S5 (light membranes and cytosol; n = 3; quantified relative to untreated [lane 1] in the experiment shown in H or across experiments in I). D, F, and I represent combined data (mean + SD) from n biological replicates. In E, G, and H, one representative example of n technical replicates is shown. *, P < 0.05 according to one-way ANOVA with Šidák correction (I; relative to respective LPS only) or two-way ANOVA (D). Citr. synth., citrate synthetase; Ctrl, control; IB, immunoblot; Nig, nigericin; Quant., quantification.