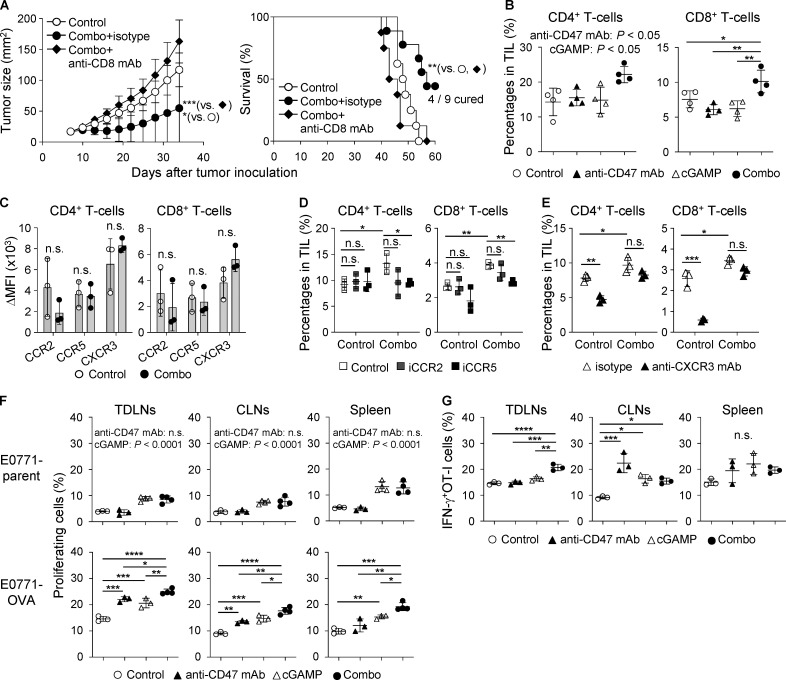
Figure 4.
Enhanced CD8+ T cell responses in mice treated with combination therapy. (A) E0771-bearing WT mice were treated with intratumoral injection of anti-CD47 mAb and/or cGAMP on day 7 after tumor inoculation. Isotype control or anti-CD8 mAb was injected i.p. into mice on days 6, 13, and 20. Tumor size (left) and survival rates (right) are shown (n = 8–9 mice/group). Data represent two independent experiments. (B–E) After 48 h of intratumoral treatment, each tumor tissue was collected for T cell analysis (n = 3–4; data are representative of two independent experiments). Percentage of CD4+ and CD8+ T cells (B) and their expression levels of chemokine receptors (C) were evaluated using a flow cytometer. ΔMFI against isotype control was calculated (target MFI minus control). Inhibitors for CCR2 and CCR5 (iCCR2 and iCCR5, respectively) and their vehicle control were i.p. injected into mice followed by intratumoral treatments (D). Blocking antibody for CXCR3 (anti-CXCR3 mAb) was i.p. administered to mice 2 h before intratumoral treatment. Isotype antibody was used as a control (E). (F and G) E0771-parent– or E0771-OVA–bearing WT mice were treated with intratumoral injections of anti-CD47 mAb and/or cGAMP on day 7 after tumor inoculation, following the transfer of CD45.1+OT-I cells. 4 d later, TDLNs, CLNs, and spleen were collected. Proliferation (F) and IFN-γ production (G) of the transferred OT-I cells were assessed (n = 3–4; data are representative of two independent experiments). Data are shown as mean ± SD. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001; two-way (A, left; B, E, F, and G) and one-way (D) ANOVA with interaction followed by Tukey’s multiple comparison test, unpaired t test (C), and log-rank (Mantel–Cox) test (A, right).