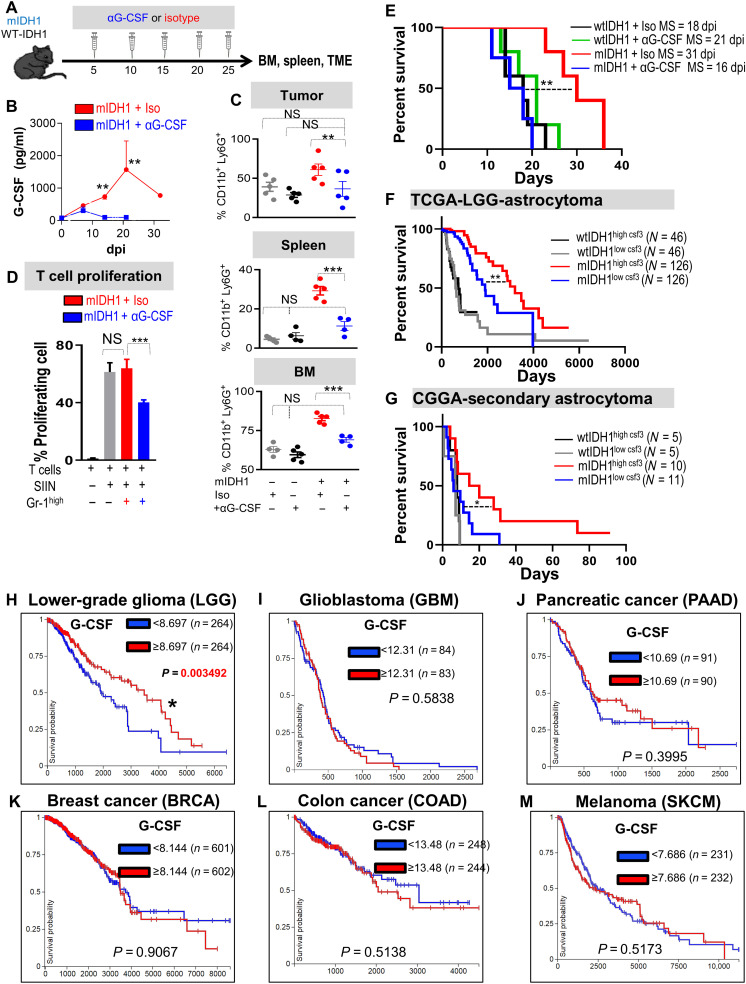
Fig. 9. G-CSF neutralization restores the immunosuppressive properties in myeloid cells and enhances the efficacy of immunotherapy.
(A) Schematic showing experimental design of G-CSF neutralization in wtIDH1 and mIDH1 tumor–bearing mice. (B) Quantitative ELISA of serum G-CSF from mIDH1 glioma-bearing mice treated with either isotype (red) or αG-CSF (blue). (C) Flow analysis of CD45high/CD11b+/Ly6G+ cells from tumor, spleen, and BM of tumor-bearing mice treated with either isotype or αG-CSF. (D) T cell proliferation assay of CD45high/CD11b+/Ly6G+ infiltrating mIDH1 tumor treated with isotype or αG-CSF. (E) Kaplan-Meier survival analysis of mice implanted with either wtIDH1 or mIDH1 neurospheres treated with isotype or αG-CSF. (F) Kaplan-Meier survival analysis of TCGA-LGG astrocytoma wtIDH1 or mIDH1 patients with high and low CSF3 expression. (G) Kaplan-Meier survival analysis of Chinese Glioma Genome Atlas (CGGA)–secondary astrocytoma wtIDH1 or mIDH1 patients with high and low levels of CSF3 expression. (H to M) Kaplan-Meier survival analysis of the different types of tumors in the TCGA data according to CSF3 expression (high or low). LGG is the sole tumor within the TCGA database in which patients who express a high level of CSF3 have a favorable prognosis compared to patients with low CSF3 expression. *P < 0.05, **P < 0.01, and ***P < 0.005, ANOVA.