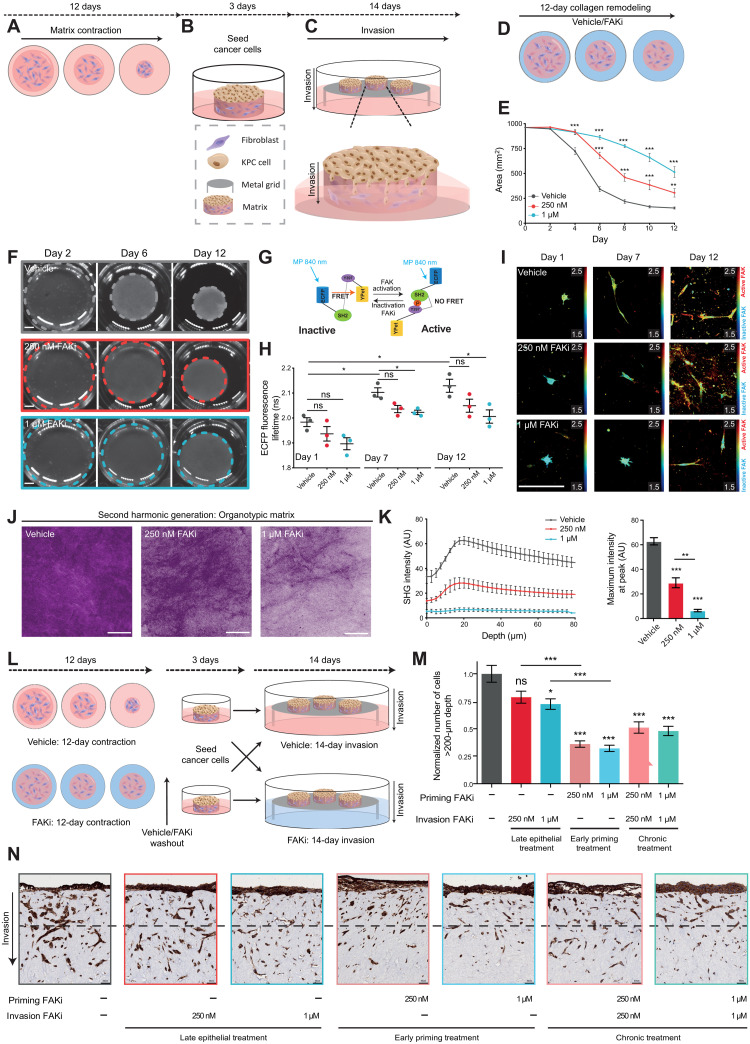
Fig. 3. Stromal FAKi priming reduces fibroblast-driven collagen remodeling and KPC cancer cell invasion into organotypic matrices.
(A to C) Schematic of 3D organotypic invasion assay: Fibroblast-driven collagen contraction (A), cancer cell seeding (B), and invasion into the contracted matrix toward the chemotactic gradient, created by air-liquid interface [note the culture medium meniscus (where cells remain in contact with media)] (C). (D) Schematic representation of fibroblast-mediated collagen matrix contraction upon vehicle or FAKi priming. (E and F) Quantification (E) and representative images (F) (scale bars, 5 mm) of matrix contraction over time. (G) Schematic of the FAK-FRET biosensor. (H and I) Quantification (H) and representative intensity-merged maps of ECFP fluorescence lifetime (I) in fibroblasts during matrix remodeling. Scale bar, 100 μm. (J and K) Representative maximum intensity SHG projections in fibroblast-contracted matrices (J) (scale bars, 100 μm) with quantification of SHG signal intensity (K) at peak (left) and over depth (in μm; right). (L) Schematic representation of organotypic invasion assay with late epithelial treatment (during KPC cell invasion), FAKi priming (during matrix contraction), and chronic treatment (during matrix contraction and invasion). (M and N) Quantification of KPC cell invasion (M) (normalized cell number at >200-μm depth) with representative images of pan-cytokeratin–stained KPC cells (N) (dotted line, 200-μm invasion depth; scale bars, 50 μm). n = 3 biological repeats, with three matrices per repeat and three FOVs per matrix (D to N). n = 20 cells per condition per replicate (H and I). Results: means ± SEM. P values were determined using an ordinary one-way ANOVA with Tukey correction for multiple comparisons. Unless otherwise stated, all significance is compared to vehicle. ns, P > 0.05; *P < 0.05, **P < 0.01, and ***P < 0.001.