Figure 2.
The N-terminal and central domains of nsp1 are required for translational suppression and mRNA depletion
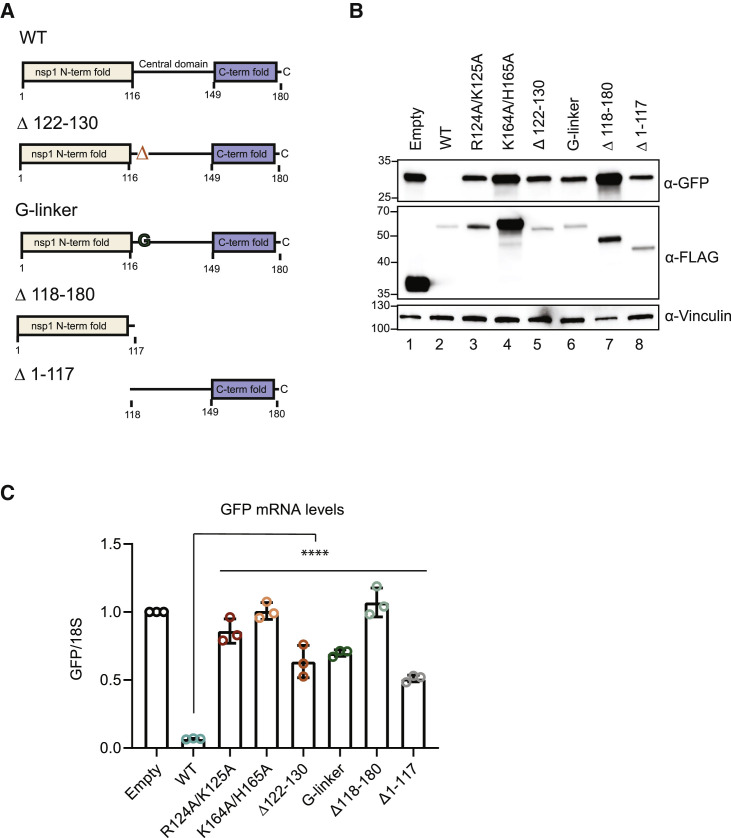
(A) Schematic of the N-terminal 3xFLAG-Halo-tagged versions of WT and mutant nsp1. Amino acids 122–130 encompass the RNA destabilization domain, which was either deleted (Δ122–130) or replaced with a size-matched glycine linker (G-linker). Mutant Δ118–180 lacks the central and C-terminal domains, whereas Δ1–117 lacks the N-terminal domain.
(B and C) HEK293T cells were transfected with a GFP reporter plasmid alone or together with plasmids containing WT or the indicated mutant nsp1 and then harvested for protein or RNA. GFP, and nsp1 protein levels were measured by ⍺-GFP and ⍺-FLAG western blots, respectively, with vinculin used as a protein loading control (B). GFP mRNA was quantified by qRT-PCR and normalized to 18S rRNA, with the level of GFP mRNA in cells lacking nsp1 then set to 1 (C). Each dot represents an independent experiment. ∗∗∗∗p ≤ 0.0001; one-way ANOVA followed by Dunnett’s multiple comparisons test versus WT nsp1. See also Figure S2. The bars represent the mean value of the replicates and error bars represent standard deviation.