Figure 6.
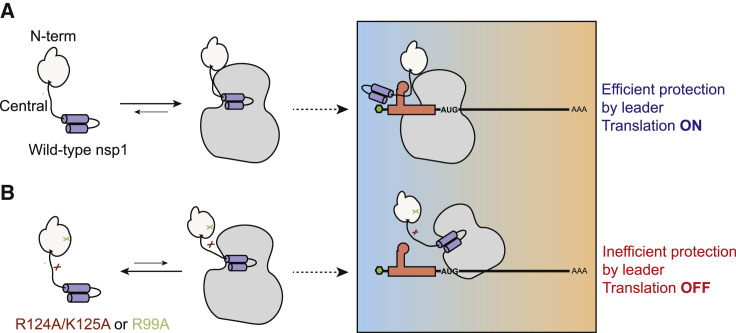
Model for how the N-terminal and central domains of nsp1 are critical for 40S ribosome association and preservation of leader-containing transcripts
(A) All three nsp1 domains contribute to its interaction with the 40S ribosome. While the C-terminal domain interjects into the mRNA entry channel of the ribosome to block mRNA access, the N-terminal and central domains stabilize the interaction. When cellular mRNA encounters an nsp1-bound ribosome, it is translationally blocked and undergoes degradation. However, mRNA containing the CoV-2 leader sequence engages the N-terminal and central domains of 40S-bound nsp1 in a manner involving nsp1 residues R124, K125, and R99, leading to relief from translational repression.
(B) Nsp1 mutants R124A/K125A and R99A have reduced affinity for the 40S ribosome, which alleviates the translational repression of cellular transcripts. However, CoV-2 leader-containing transcripts instead become translationally repressed, perhaps due to a “nonproductive” interaction with nsp1 in the absence of proper engagement with residues R124/K125 or R99.