Abstract
Objective
This study aims to comprehensively evaluate olfactory and gustatory dysfunctions during the COVID-19 pandemic regarding onset, course, associated symptoms, prognosis and relation to patients' demographics, treatment received and other symptoms.
Patients& methods
This is a prospective study conducted on patients proven to be infected with COVID-19 and with olfactory/gustatory dysfunction symptoms. Detailed history was taken from each patient about the onset of this dysfunction, associated symptoms. Then follow-up survey was done after 6 months to evaluate the prognosis.
Results
1031 patients were included in the study, aged 18 to 69 years old, with 31.8% were male. Olfactory/gustatory dysfunctions occurred after other COVID-19 symptoms in 43.5% of cases, occurred suddenly in 80.4% and gradually in 19.6%. These dysfunctions were anosmia & ageusia in 50.2%, hyposmia & hypogeusia in 23.3%, anosmia alone in 17.7%, phantosmia in 18%, Parosmia in 28.4%. In terms of recovery 6-month follow up, 680 patients (66%) recovered completely, 22.1% recovered partially while 11.9% did not recover. Most improvement occurred in the first two weeks. Headache, malaise, nasal obstruction and rhinorrhea were the commonest COVID-19 symptoms associated.
Conclusion
Most recovery of olfactory/gustatory dysfunction in COVID-19 infection occurs at the first two weeks and is unrelated to patient demographics, treatment or olfactory training. Parosmia is an independent predictor for complete recovery, while phantosmia is significantly associated with lower probability of complete recovery.
Keywords: COVID-19, Anosmia, Hyposmia, Dysgeusia, SARS-CoV-2
1. Introduction
The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) began and spread in China by the end of 2019 after that received worldwide attention. By the end of January 2020, WHO officially declared Coronavirus disease 2019 (COVID-19) epidemic as a public health emergency of international concern and as a pandemic in March 2020. Since then, COVID-19 hits every corner of the world [1]. Anosmia and taste disorders are considered main symptoms associated with the COVID-19 infection and as potential screening symptoms for suspecting and testing for COVID-19 [2], [3]. Nasal respiratory and olfactory cells express Angiotensin Converting Enzyme 2 proteins which are used by the COVID-19 virus to infect cells. Also, some strains of coronavirus could invade the olfactory bulb [4]. Although olfactory and gustatory dysfunctions present as symptoms in 18 to 60% of COVID-19 patients and patients presenting with anosmia and influenza-like symptoms are 6–10 times more likely to be COVID-19 positive [5], [6], there is no sufficient data in the literature regarding the course, recovery, associated symptoms and prognosis of olfactory and gustatory symptoms. Our study aims to document all these missing data comprehensively using more than one thousand patient data.
2. Patients & methods
This is a prospective study for olfactory and gustatory dysfunctions in COVID-19 patients evaluated at tertiary referral center and confirmed to be positive by PCR of nasopharyngeal swab from 1st August 2020 to 31st October 2020. Inclusion criteria included patients with mild to moderate adult COVID-19 patients who presented with olfactory and/or gustatory dysfunctions. All patients with history of nasal or oral surgery or trauma, chronic rhinosinusitis, previous history (before the pandemic) of olfactory or gustatory functions were excluded.
Detailed history was taken from each patient by physicians or nurses completing sheets to document the onset of olfactory and gustatory dysfunction and associated symptoms. Then follow up survey was done after 6 months by phone call or physically to document the progression of olfactory and gustatory dysfunction and their prognosis. Data collection was conducted anonymously, and no reward was offered for completion.
The data involved demographics, smoking history, history of contact, medical comorbidities, associated COVID-19 symptoms. As regards olfactory and gustatory dysfunctions, it included data about the presented form of dysfunction, time of onset, course, duration, time of recovery, treatment received, olfactory training usage, prognosis.
This study was approved by Ain Shams University, Faculty of Medicine institutional review board. Patients were invited to participate. All patients signed informed written consent prior study started. All patients' data were dealt with complete confidentiality.
3. Statistical methods
Data were analyzed using IBM© SPSS© Statistics version 26 (IBM© Corp., Armonk, NY). Categorical variables are presented as ratios or numbers and percentages and differences are compared using the Pearson chi-squared test or Fisher's exact test as appropriate. Ordinal data are compared using the chi-squared test for trends.
Multivariable binary logistic regression analysis is used to identify predictors of complete recovery. Predictors shown by bivariate analysis to be associated with the outcome at a level of p ≤ 0.2 were entered in multiple regression. We constructed the model using the enter method. P-values <0.05 are considered statistically significant.
4. Results
1031 patients completed all the data needed and the follow-up, aged from 18 to 69 years old. Almost a third (32.4%) of these cases were in contact with confirmed COVID-19 cases. Abnormal smell presented in 97.9% of these cases while abnormal taste was in 75.7%. Anosmia presented in 67.9%, hyposmia in 30%, phantosmia in 18.0%, parosmia in 28.4%. In 43.5% of cases, smell/taste abnormalities occurred after COVID-19 symptoms. Complete recovery of smell/taste changes occurred in 66% of cases, while partial recovery occurred in 22.1%. 70.1% of cases reached the best recovery in the first two weeks (Table 1 ).
Table 1.
Characteristics of the study population.
| Variable | Count | Valid percentage |
|---|---|---|
| Age category (years) | ||
| 18–20 | 45 | 4.4% |
| 21–30 | 524 | 50.9% |
| 31–40 | 344 | 33.4% |
| 41–50 | 97 | 9.4% |
| 51–60 | 17 | 1.7% |
| 61–70 | 3 | 0.3% |
| Sex | ||
| M | 328 | 31.8% |
| F | 703 | 68.2% |
| Past history | ||
| Smoking | 136 | 13.2% |
| Medical comorbidities | 104 | 10.1% |
| Contact with confirmed COVID-19 case | 334 | 32.4% |
| Sought prior medical advice | 791 | 76.7% |
| General manifestations of COVID-19 | ||
| Dry cough | 178 | 17.3% |
| Product cough | 158 | 15.3% |
| Dyspnea | 194 | 18.8% |
| Rhinorrhea/nasal obstruction | 316 | 30.6% |
| Malaise | 505 | 49.0% |
| Diarrhea | 250 | 24.2% |
| Nausea | 170 | 16.5% |
| Fever | 277 | 26.9% |
| Sore throat | 272 | 26.4% |
| Headache | 502 | 48.7% |
| Abdominal pain | 241 | 23.4% |
| Presentation of the smell/taste disorder | ||
| Hyposmia only | 69 | 6.7% |
| Hypogeusia only | 11 | 1.1% |
| Hyposmia & hypogeusia | 240 | 23.3% |
| Anosmia only | 182 | 17.7% |
| Ageusia only | 11 | 1.1% |
| Anosmia & ageusia | 518 | 50.2% |
| Occurrence of complex forms of smell diorders | ||
| Phantosmia | ||
| Did not occur | 845 | 82.0% |
| Occurred transiently then recovered | 166 | 16.1% |
| Occurred and did not recover | 20 | 1.9% |
| Parosmia | ||
| Did not occur | 738 | 71.6% |
| Occurred transiently then recovered | 251 | 24.3% |
| Occurred and did not recover | 42 | 4.1% |
| Overall prevalence of individual forms of smell/taste abnormality | ||
| Hyposmia | 309 | 30.0% |
| Hypogeusia | 251 | 24.3% |
| Anosmia | 700 | 67.9% |
| Ageusia | 529 | 51.3% |
| Abnormal smell (hyposmia or anosmia) | 1009 | 97.9% |
| Abnormal taste (hypogeusia or ageusia) | 780 | 75.7% |
| Phantosmia | 186 | 18.0% |
| Parosmia | 293 | 28.4% |
| Onset of smell/taste changes | ||
| Gradual | 202 | 19.6% |
| Sudden | 829 | 80.4% |
| Onset of smell/taste changes in relation to COVID-19 symptoms | ||
| Before COVID-19 symptoms | 200 | 19.4% |
| During COVID-19 symptoms | 383 | 37.1% |
| After COVID-19 symptoms | 448 | 43.5% |
| Treatment received for smell/taste changes | ||
| Intranasal steroids | 312 | 30.3% |
| Systemic steroids | 62 | 6.0% |
| Omega-3 FA | 104 | 10.1% |
| Others | 305 | 29.6% |
| Combination of medications | 41 | 4.0% |
| Nil | 207 | 20.1% |
| Awareness of patient about smell training | ||
| Not aware about it | 569 | 55.2% |
| Aware, does not practice it | 195 | 18.9% |
| Aware, practices it inappropriately | 103 | 10.0% |
| Aware, practices it appropriately | 164 | 15.9% |
| Recovery of smell/taste | ||
| Not recovered | 123 | 11.9% |
| Recovered partially | 228 | 22.1% |
| Recovered completely | 680 | 66.0% |
| Time to best recovery (for those experiencing improvement) | ||
| <1 week | 310/908 | 34.1% |
| 1-<2 weeks | 327/908 | 36.0% |
| 2-<3 weeks | 104/908 | 11.5% |
| 3-<4 weeks | 63/908 | 6.9% |
| 1-<2 months | 21/908 | 2.3% |
| 2-<3 months | 41/908 | 4.5% |
| 3–6 months | 42/908 | 4.6% |
After adjustment for the effect of other factors, parosmia was an independent predictor for complete recovery (odds ratio = 1.787, 95% CI = 1.304 to 2.449, p-value = 0.0003). On the other hand, phantosmia was significantly associated with lower probability of complete recovery (odds ratio = 0.281, 95% CI = 0.200 to 0.395, p-value <0.0001). (Table 2, Table 3, Table 4 , Fig. 1, Fig. 2, Fig. 3 ).
Table 2.
Predictors of improvement.
| Variable | No recovery (n = 123) |
Partial or complete recovery (n = 908) |
p-Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age category (years) | 0.449a | ||||
| 18–20 | 4 | 3.3% | 41 | 4.5% | |
| 21–30 | 68 | 55.3% | 456 | 50.3% | |
| 31–40 | 41 | 33.3% | 303 | 33.4% | |
| 41–50 | 7 | 5.7% | 90 | 9.9% | |
| 51–60 | 3 | 2.4% | 14 | 1.5% | |
| 61–70 | 0 | 0.0% | 3 | 0.3% | |
| Sex | 0.700b | ||||
| M | 41 | 33.3% | 287 | 31.6% | |
| F | 82 | 66.7% | 621 | 68.4% | |
| Past history | |||||
| Smoking | 19 | 15.4% | 117 | 12.9% | 0.431b |
| Medical comorbidities | 14 | 11.4% | 90 | 9.9% | 0.611b |
| Contact with confirmed COVID-19 case | 41 | 33.3% | 293 | 32.3% | 0.813b |
| Sought prior medical advice | 93 | 75.6% | 698 | 76.9% | 0.756b |
| General manifestations of COVID-19 | |||||
| Dry cough | 15 | 12.2% | 163 | 18.0% | 0.113b |
| Product cough | 16 | 13.0% | 142 | 15.6% | 0.447b |
| Dyspnea | 21 | 17.1% | 173 | 19.1% | 0.598b |
| Rhinorrhea/nasal obstruction | 32 | 26.0% | 284 | 31.3% | 0.235b |
| Malaise | 54 | 43.9% | 451 | 49.7% | 0.230b |
| Diarrhea | 25 | 20.3% | 225 | 24.8% | 0.279b |
| Nausea | 15 | 12.2% | 155 | 17.1% | 0.171b |
| Fever | 25 | 20.3% | 252 | 27.8% | 0.081b |
| Sore throat | 34 | 27.6% | 238 | 26.2% | 0.735b |
| Headache | 56 | 45.5% | 446 | 49.1% | 0.455b |
| Abdominal pain | 24 | 19.5% | 217 | 23.9% | 0.281b |
| Presentation of the smell/taste disorder | 0.268c | ||||
| Hyposmia only | 6 | 4.9% | 63 | 6.9% | |
| Hypogeusia only | 2 | 1.6% | 9 | 1.0% | |
| Hyposmia & hypogeusia | 28 | 22.8% | 212 | 23.3% | |
| Anosmia only | 30 | 24.4% | 152 | 16.7% | |
| Ageusia only | 0 | 0.0% | 11 | 1.2% | |
| Anosmia & ageusia | 57 | 46.3% | 461 | 50.8% | |
| Overall prevalence of individual forms of smell/taste abnormality | |||||
| Hyposmia | 34 | 27.6% | 275 | 30.3% | 0.548b |
| Hypogeusia | 30 | 24.4% | 221 | 24.3% | 0.990b |
| Anosmia | 87 | 70.7% | 613 | 67.5% | 0.473b |
| Ageusia | 57 | 46.3% | 472 | 52.0% | 0.240b |
| Abnormal smell | 121 | 98.4% | 888 | 97.8% | 1.000c |
| Abnormal taste | 87 | 70.7% | 693 | 76.3% | 0.175b |
| Phantosmia | 20 | 16.3% | 166 | 18.3% | 0.584b |
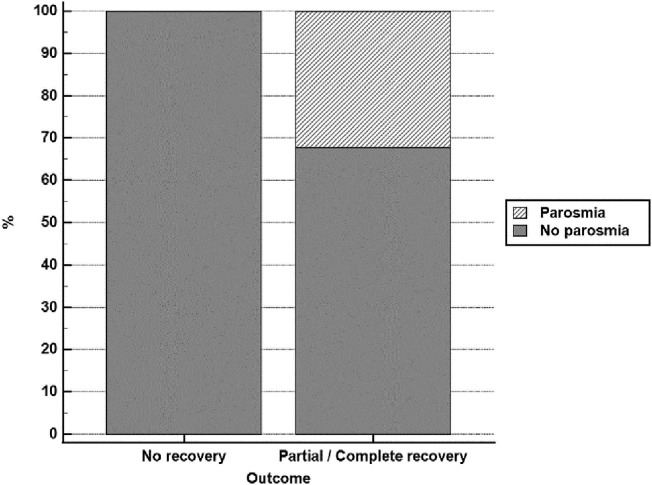
| Parosmia | 0 | 0.0% | 293 | 32.3% | <0.001b |
| Onset of smell/taste changes | 0.483b | ||||
| Gradual | 27 | 22.0% | 175 | 19.3% | |
| Sudden | 96 | 78.0% | 733 | 80.7% | |
| Onset of smell/taste changes in relation to COVID-19 symptoms | 0.941a | ||||
| Before COVID-19 symptoms | 23 | 18.7% | 177 | 19.5% | |
| During COVID-19 symptoms | 48 | 39.0% | 335 | 36.9% | |
| After COVID-19 symptoms | 52 | 42.3% | 396 | 43.6% | |
| Treatment received for smell/taste changes | |||||
| Intranasal steroids | 39 | 31.7% | 273 | 30.1% | 0.710b |
| Systemic steroids | 4 | 3.3% | 58 | 6.4% | 0.170b |
| Omega-3 FA | 13 | 10.6% | 91 | 10.0% | 0.850b |
| Others | 36 | 29.3% | 269 | 29.6% | 0.935b |
| Combination of medications | 4 | 3.3% | 37 | 4.1% | 0.809c |
| Nil | 27 | 22.0% | 180 | 19.8% | 0.580b |
| Smell training | 0.578a | ||||
| Not aware about it | 76 | 61.8% | 493 | 54.3% | |
| Aware, does not practice it | 15 | 12.2% | 180 | 19.8% | |
| Aware, practices it inappropriately | 11 | 8.9% | 92 | 10.1% | |
| Aware, practices it appropriately | 21 | 17.1% | 143 | 15.7% | |
Data are number (N) and percentage (%).
Bold means a statistical significance.
Pearson chi-squared test.
Fisher's exact test.
Chi-squared test for trend.
Table 3.
Predictors of complete recovery.
| Variable | No or partial recovery (n = 351) |
Complete recovery (n = 680) |
p-Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age category (years) | 0.966a | ||||
| 18–20 | 13 | 3.70% | 32 | 4.70% | |
| 21–30 | 182 | 51.90% | 342 | 50.40% | |
| 31–40 | 118 | 33.60% | 226 | 33.30% | |
| 41–50 | 30 | 8.50% | 67 | 9.90% | |
| 51–60 | 7 | 2.00% | 10 | 1.50% | |
| 61–70 | 1 | 0.30% | 2 | 0.30% | |
| Sex | 0.173b | ||||
| M | 102 | 29.10% | 226 | 33.20% | |
| F | 249 | 70.90% | 454 | 66.80% | |
| Past history | |||||
| Smoking | 47 | 13.40% | 89 | 13.10% | 0.892b |
| Medical comorbidities | 37 | 10.50% | 67 | 9.90% | 0.728b |
| Contact with confirmed COVID-19 case | 120 | 34.20% | 214 | 31.50% | 0.377b |
| Sought prior medical advice | 260 | 74.10% | 531 | 78.10% | 0.148b |
| General manifestations of COVID-19 | |||||
| Dry cough | 60 | 17.10% | 118 | 17.40% | 0.917b |
| Product cough | 56 | 16.00% | 102 | 15.00% | 0.687b |
| Dyspnea | 69 | 19.70% | 125 | 18.40% | 0.619b |
| Rhinorrhea/nasal obstruction | 110 | 31.30% | 206 | 30.30% | 0.730b |
| Malaise | 170 | 48.40% | 335 | 49.30% | 0.800b |
| Diarrhea | 76 | 21.70% | 174 | 25.60% | 0.162b |
| Nausea | 53 | 15.10% | 117 | 17.20% | 0.388b |
| Fever | 95 | 27.10% | 182 | 26.80% | 0.918b |
| Sore throat | 91 | 25.90% | 181 | 26.60% | 0.811b |
| Headache | 165 | 47.00% | 337 | 49.60% | 0.438b |
| Abdominal pain | 80 | 22.80% | 161 | 23.70% | 0.751b |
| Presentation of the smell/taste disorder | 0.178c | ||||
| Hyposmia only | 18 | 5.10% | 51 | 7.50% | |
| Hypogeusia only | 5 | 1.40% | 6 | 0.90% | |
| Hyposmia & hypogeusia | 87 | 24.80% | 153 | 22.50% | |
| Anosmia only | 73 | 20.80% | 109 | 16.00% | |
| Ageusia only | 4 | 1.10% | 7 | 1.00% | |
| Anosmia & ageusia | 164 | 46.70% | 354 | 52.10% | |
| Overall prevalence of individual forms of smell/taste abnormality | |||||
| Hyposmia | 105 | 29.90% | 204 | 30.00% | 0.977b |
| Hypogeusia | 92 | 26.20% | 159 | 23.40% | 0.316b |
| Anosmia | 237 | 67.50% | 463 | 68.10% | 0.853b |
| Ageusia | 168 | 47.90% | 361 | 53.10% | 0.112b |
| Abnormal smell | 342 | 97.40% | 667 | 98.10% | 0.492b |
| Abnormal taste | 260 | 74.10% | 520 | 76.50% | 0.396b |
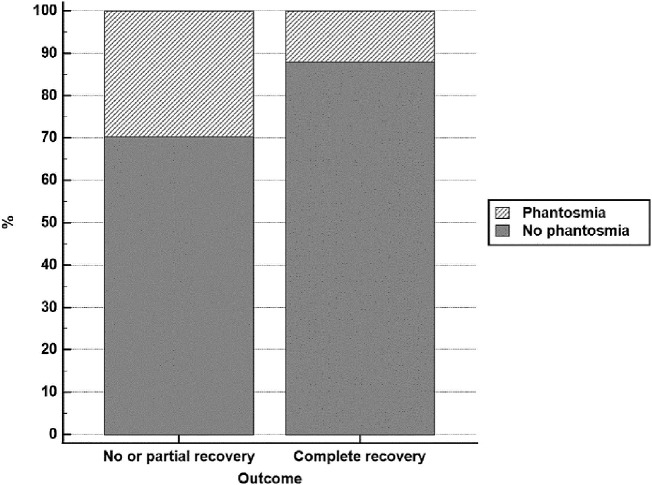
| Phantosmia | 104 | 29.60% | 82 | 12.10% | <0.001b |
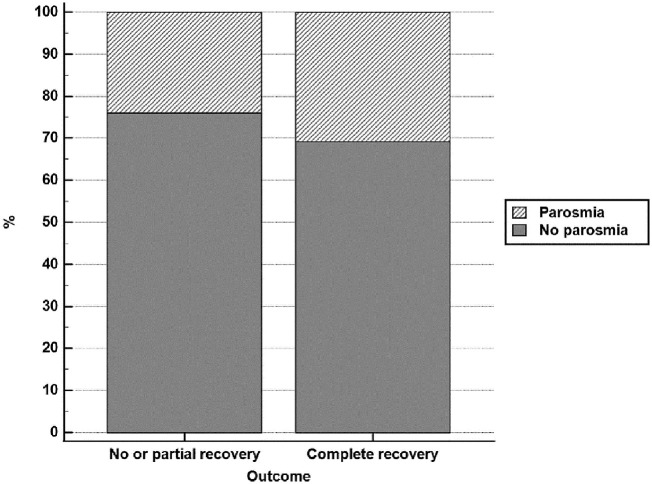
| Parosmia | 84 | 23.90% | 209 | 30.70% | 0.022b |
| Onset of smell/taste changes | 0.839b | ||||
| Gradual | 70 | 19.90% | 132 | 19.40% | |
| Sudden | 281 | 80.10% | 548 | 80.60% | |
| Onset of smell/taste changes in relation to COVID-19 symptoms | 0.628a | ||||
| Before COVID-19 symptoms | 66 | 18.80% | 134 | 19.70% | |
| During COVID-19 symptoms | 129 | 36.80% | 254 | 37.40% | |
| After COVID-19 symptoms | 156 | 44.40% | 292 | 42.90% | |
| Treatment received for smell/taste changes | |||||
| Intranasal steroids | 112 | 31.90% | 200 | 29.40% | 0.408b |
| Systemic steroids | 19 | 5.40% | 43 | 6.30% | 0.560b |
| Omega-3 FA | 36 | 10.30% | 68 | 10.00% | 0.897b |
| Others | 107 | 30.50% | 198 | 29.10% | 0.649b |
| Combination of medications | 12 | 3.40% | 29 | 4.30% | 0.510b |
| Nil | 65 | 18.50% | 142 | 20.90% | 0.369b |
| Patient awareness of smell training | 0.145a | ||||
| Not aware about it | 207 | 59.00% | 362 | 53.20% | |
| Aware, does not practice it | 62 | 17.70% | 133 | 19.60% | |
| Aware, practices it inappropriately | 29 | 8.30% | 74 | 10.90% | |
| Aware, practices it appropriately | 53 | 15.10% | 111 | 16.30% | |
Data are number (N) and percentage (%).
Bold means a statistical significance.
Pearson chi-squared test.
Fisher's exact test.
Chi-squared test for trend.
Table 4.
Multivariable binary logistic regression for predictors of complete recovery.
| Variable | B | SE | Wald | p-Value | Odds ratio | 95% CI |
|---|---|---|---|---|---|---|
| Female sex (=1) | −0.234 | 0.148 | 2.485 | 0.115 | 0.792 | 0.592 to 1.059 |
| Seeking prior medical advice (=1) | 0.173 | 0.159 | 1.190 | 0.275 | 1.189 | 0.871 to 1.624 |
| Diarrhea (=1) | 0.252 | 0.162 | 2.435 | 0.119 | 1.287 | 0.938 to 1.767 |
| Ageusia (=1) | 0.240 | 0.136 | 3.088 | 0.079 | 1.271 | 0.973 to 1.661 |
| Phantosmia (=1) | −1.271 | 0.174 | 53.303 | <0.0001 | 0.281 | 0.200 to 0.395 |
| Parosmia (=1) | 0.580 | 0.161 | 13.029 | 0.0003 | 1.787 | 1.304 to 2.449 |
| Constant | 0.604 | 0.195 | 9.586 | 0.002 |
B = regression coefficient, SE = standard error, Wald = Wald statistics, 95% CI = 95% confidence interval for odds ratio.
Fig. 1.
Prevalence of parosmia among patients experiencing partial or complete recovery versus those experiencing no recovery.
Fig. 2.
Prevalence of phantosmia among patients experiencing complete recovery versus those experiencing no or partial recovery.
Fig. 3.
Prevalence of parosmia among patients experiencing complete recovery versus those experiencing no or partial recovery.
5. Discussion
COVID-19 disease rapidly spreads across every corner world. Otorhinolaryngologists may be in the front line due to the close contact with the mucus membrane of the upper respiratory tract. Olfactory and gustatory dysfunctions are very characteristic symptoms of the disease. So, this study is primarily concerned with olfactory and gustatory dysfunctions during the pandemic, comprehensively evaluating the onset, course and relation to the COVID-19 course and its symptoms. This study was done in a pandemic hospital on confirmed COVID-19 adult patients by PCR, who had symptoms of olfactory and gustatory dysfunctions. 1031 patients were included in our study, aged 18 to 69 years old, with 31.8% were male. 86.8% were non-smokers, 89.9% had no comorbidities. 32.4% of these cases were in contact with confirmed COVID-19 cases, and 76.7% sought prior medical advice.
In their study on 268 patients, Oscolo-Rizzo et al. reported interquartile range of age was 38–56 years with female preponderance 61.9%, co morbidities were reported in 34.0% [7]. Hopkins et al. did their study on 382 patients, age ranged between 18 and 79 years old, 74.6% were female [4]. Lechien et al. did their study on 1363 patients aged 41.9 ± 13.0 years old, 62.9% were female, 11.4% were smokers [8].
In our study, baseline sociodemographic and lifestyle factors were not associated with olfactory/taste dysfunction persistence. This agrees with Oscolo-Rizzo et al. [7].
In our study, olfactory/gustatory dysfunctions occurred before other COVID-19 symptoms in 19.4% of cases, with other COVID-19 symptoms in 37.1% and after in 43.5%. Olfactory/gustatory dysfunctions occurred suddenly in 80.4% and gradually in 19.6%. These dysfunctions were anosmia & ageusia in 518 patients (50.2%), hyposmia & hypogeusia in 240 (23.3%), anosmia alone in 17.7%, hyposmia alone in 6.7%, hypogeusia alone in 1.1%, ageusia alone in 1.1%. Phantosmia occurred in 186 cases (18%), 16.1% of them recovered. Parosmia occurred in 28.4%, of which 24.3% recovered.
According to Lechien et al., anosmia formed 81.6% of the cases while hyposmia formed 18.4%. Dysgeusia was 55.9%. Phantosmia formed 16.4%. Olfactory dysfunction developed after other COVID-19 symptoms in 44.7%, before in 16.8% [8]. In their study, Hopkins et al. found 86.4% with anosmia and 11.5% with hyposmia. 14.9% reported smell changes before the onset of other COVID-19 symptoms, 39.3% at the same time and 45.8% after [4].
According to Oscolo-Rizzo et al., 81.3% reported combined olfactory/taste dysfunctions, 10.2% reported isolated smell impairment, 8.6% reported isolated taste disorder [7].
In terms of recovery of olfactory/gustatory dysfunctions after 6-month follow up, 680 patients (66%) recovered completely, 22.1% recovered partially while 11.9% did not recover. Most improvement occurred in the first two weeks (in 637 /908 patients (70.1%)). This agrees with Hopkins et al. who stated a significant recovery rate in the first 2 weeks but then it became plateau [4].
Oscolo-Rizzo et al. reported 69.5% complete recovery after 12 months, 21.9% partial recovery and 8.6% no improvement [7]. Hopkins et al. stated improvement rate was 79% while 17.3% had persistent anosmia after 4 weeks of follow up [4].
According to Lechien et al., over one third of the patients recovered from olfactory dysfunction in the first two weeks, 54.3% recovered after one month. 24.5% did not recove after two months. Recovery rate ranges from 75% to 85% in the first two months [8].
In terms of other COVID-19 symptoms in patients in the current study, headache occurred in 48.7%, malaise in 49%, rhinorrhea/nasal obstruction in 30.6%, fever in 26.9%, cough in 32.6%, sore throat in 26.4%. According to Lechien et al., the most common associated COVID-19 symptoms were asthenia 86.3%, headache 69.9% and rhinorrhea 64.4%, nasal obstruction 62.1% [8].
In our study, 30.3% received intranasal steroids, 6% received systemic steroids, 10.1% received Omega-3 fatty acids while 20.1% did not receive any treatment. 55.2% of patients were not aware of olfactory training, 18.9% were aware, but didn't not practice it. 10% were aware but practiced it inappropriately. 15.9% were aware and practiced it appropriately. But there was no statistically significant association between using olfactory training and recovery. This agrees with Hopkins et al. [4].
In their study on 30 patients with olfactory and gustatory dysfunction, Konstantinidis et al. found smell and taste changes in 70%, only smell changes in 26.6%, only taste changes in 3.3%. 63.3% recovered completely, 36.6% with partial or no recovery after 4 weeks of follow up. Nasal obstruction was reported in 16.6%, rhinorrhea in 10%, parosmia and phantosmia in 13%, dysgeusia in 10% [9]. Renaud et al. concluded a 96.1% recovery rate from COVID-19 olfactory dysfunction after 1 year of follow up. [10]
Olfactory dysfunction may have different course and progression in COVID-19 patients. According to studies, this is due to differences in the expression of ACE2 between individuals. The more expression of these proteins is mostly associated with longer duration of smell changes and more injury to stem neuron cells in the olfactory bulb. [11], [12]
By analyzing every factor in the current study in terms of improvement of olfactory/gustatory dysfunctions or complete recovery after adjustment for the effect of other factors, we found a statistically significant association between parosmia and partial or complete recovery. Also, parosmia was an independent predictor for complete recovery while phantosmia was significantly associated with lower probability of complete recovery.
6. Conclusion
Most recovery of olfactory/gustatory dysfunction in COVID-19 infection occurs at the first two weeks and is unrelated to patient demographics, treatment or olfactory training. Parosmia is an independent predictor for complete recovery while phantosmia is significantly associated with lower probability of complete recovery.
Financial disclosure
The authors have no financial sponsorship to disclose.
The manuscript wasn't presented in any conference or meeting.
Declaration of competing interest
The authors have no conflict of interest to disclose.
Footnotes
Institution at which study was performed: Ain Shams University Hospitals, Cairo, Egypt.
References
- 1.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., Tan K.S., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020 Mar 13;7(1):11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giacomelli A., Pezzati L., Conti F., Bernacchia D., Siano M., Oreni L., Rusconi S., et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 2020 Jul 28;71(15):889–890. doi: 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020 Aug;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins C., Surda P., Whitehead E., Kumar B.N. Early recovery following new onset anosmia during the COVID-19 pandemic - an observational cohort study. J Otolaryngol Head Neck Surg. 2020 May 4;49(1):26. doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and ageusia: common findings in COVID-19 patients. Laryngoscope. 2020 Jul;130(7):1787. doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lechien J.R., Chiesa-Estomba C.M., Place S., Van Laethem Y., Cabaraux P., Mat Q., Huet K., et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020 Sep;288(3):335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oscolo-Rizzo P., Guida F., Polesel J., Marcuzzo A.V., Antonucci P., Capriotti V., Sacchet E., et al. Self-reported smell and taste recovery in coronavirus disease 2019 patients: a one-year prospective study. Eur Arch Otorhinolaryngol. 2021 May;7:1–6. doi: 10.1007/s00405-021-06839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechien J.R., Chiesa-Estomba C.M., Beckers E., Mustin V., Ducarme M., Journe F., Marchant A., et al. Prevalence and 6-month recovery of olfactory dysfunction: a multicentre study of 1363 COVID-19 patients. J Intern Med. 2021 Aug;290(2):451–461. doi: 10.1111/joim.13209. [DOI] [PubMed] [Google Scholar]
- 9.Konstantinidis I., Delides A., Tsakiropoulou E., Maragoudakis P., Sapounas S., Tsiodras S. Short-term follow-up of self-isolated COVID-19 patients with smell and taste dysfunction in Greece: two phenotypes of recovery. ORL J Otorhinolaryngol Relat Spec. 2020;82(6):295–303. doi: 10.1159/000511436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renaud M., Thibault C., Le Normand F., Mcdonald E.G., Gallix B., Debry C., Venkatasamy A. Clinical outcomes for patients with anosmia 1 year after COVID-19 diagnosis. JAMA Netw Open. 2021 Jun 1;4(6) doi: 10.1001/jamanetworkopen.2021.15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen M., Shen W., Rowan N.R., Kulaga H., Hillel A., Ramanathan M., Jr., et al. Elevated ACE-2 expression in the olfactory neuroepithelium: implications for anosmia and upper respiratory SARS-CoV-2 entry and replication. Eur Respir J. 2020 Sep 24;56(3) doi: 10.1183/13993003.01948-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., Wen F., Huang X., Ning G., Wang W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020 Feb;24(6):11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]