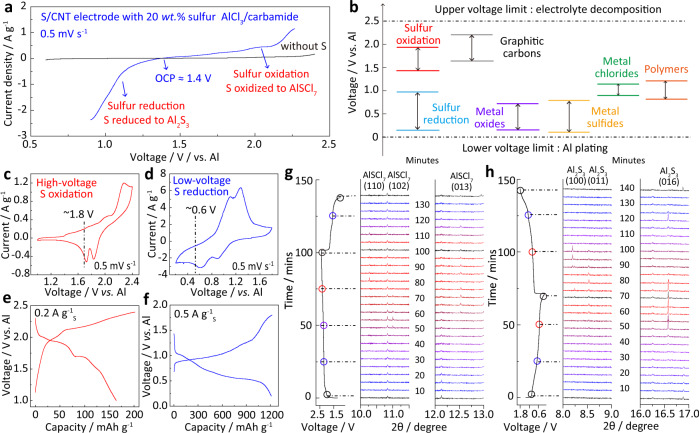
Fig. 1. The electrochemical oxidation and reduction of sulfur in ionic liquid.
a LSV curves of S/CNT composite cathode in AlCl3/carbamide ionic liquid at 0.5 mV s−1 with Al referenced electrode; the sulfur content in S/CNT is 20 wt.%; b the voltage comparison of sulfur oxidation and sulfur reduction with previously reported materials; CV curves of c sulfur oxidation and d sulfur reduction at 0.5 mV s−1; galvanostatic charge–discharge curves of the S/CNT cathode based on e sulfur oxidation at 0.2 A g−1 and f sulfur reduction at 0.5 A g−1; the time-dependent in situ synchrotron-based XRD patterns for g sulfur oxidation and h sulfur reduction processes and the corresponding charge–discharge curves. The current densities for sulfur oxidation and sulfur reduction are 0.2 and 0.5 A g−1, respectively.