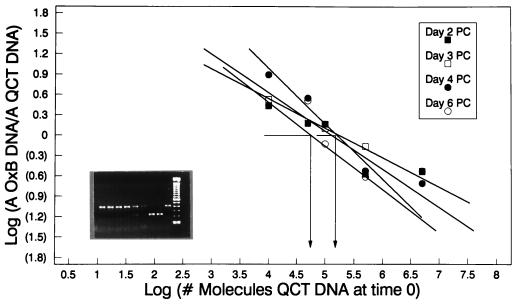
FIG. 5.
Testing clinical samples for stability and reproducibility in detecting O. formigenes. Four fecal swabs collected and transported in the Cary-Blair culture transport system were processed on days 2, 3, 4, and 6 following collection to analyze the sample stability and the reproducibility of the QC-PCR assay system. DNA was isolated from approximately 20 mg of fecal specimen eluted from each swab, and the presence of O. formigenes genomes was determined by QC-PCR (insert). Log equivalences of O. formigenes to template band intensities were graphed to quantify the number of genomes present in each sample. The number of CFU detected on days 2, 3, and 4 was 8 × 108/g (wet weight), while the number of CFU detected on day 6 was 2.8 × 108/g (wet weight). QCT, quantitative competitive template.