Abstract
Pre-operative localization of non-palpable breast lesions with non-wired non-ionizing (NWNI) techniques may improve clinical outcomes as reoperation rate, cosmetic outcome and contribute to organizational aspects improvement in breast-conserving surgery (BCS). However only limited literature is available and clinical studies involving these forefront devices are often small and non-randomized. Furthermore, there is a lack of consensus on free margins and cosmetic outcomes definitions. The objective of the present meta-analysis was to determine the crude clinical outcomes reported for the NWNI techniques on BCS. A literature search was performed of PubMed, Embase and Scopus databases up to February 2021 in order to select all prospective or retrospective clinical trials on pre-operative breast lesion localization done with NWNI devices. All studies were assessed following the PRISMA recommendations. Continuous outcomes were described in averages corrected for sample size, while binomial outcomes were described using the weighted average proportion.
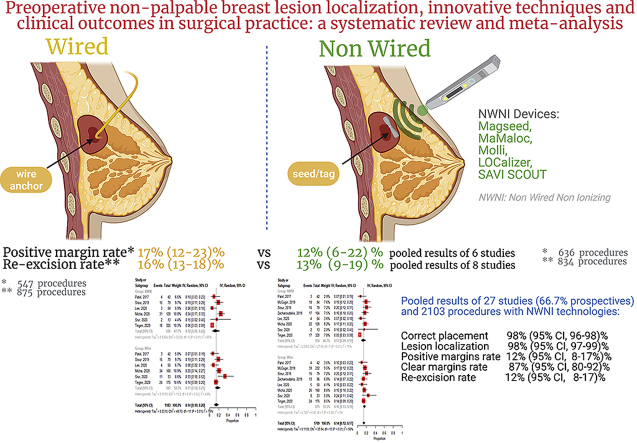
Twenty-seven studies with a total of 2103 procedures were identified. The technique is consolidated, showing for both reflectors’ positioning and localization nearly the 100% rate of success. The re-excision and clear margins rates were 14% (95% CI, 11–17%) and 87% (80–92%), respectively. Overall, positive margins rates were 12% (8–17%). In studies that compared NWNI and wire localization techniques, positive margin rate is lower for the first techniques (12%, 6–22% vs 17%, 12–23%) and re-excision rate is slightly higher using the latter (13%, 9–19% vs 16%, 13–18%).
Pre-operative NWNI techniques are effective in the localization of non-palpable breast lesions and are promising in obtaining clear (or negative) margins minimizing the need for re-excision and improving the cosmetic outcomes. Randomized trials are needed to confirm these findings.
Keywords: Breast, Non-palpable lesion, Localization, Breast-conserving surgery, Clear margin, Re-operation, Re-excison, Non-wired, Preoperative, Cosmetic outcome, Meta-analysis, Systematic review
Graphical abstract
Highlights
-
•
Positioning and localization of reflectors' is nearly of the 100% rate of success. Overall, positive margins rates were 12% (8–17%).
-
•
The re-excision and clear margins rates were 12% (95% CI, 8–17%) and 87% (80–92%), respectively.
-
•
Comparing NWNI and WGL techniques, positive margin rate is lower for the first one and re-excision rate is slightly higher using the latter.
-
•
This technology overcomes the limitations related to other techniques: migrations, the coordination between radiology and surgery, the use of radioactive substances.
-
•
The procedure is comfortable for the radiologist while allow surgeon at resecting the non-palpable lesions ensuring clear margins, avoiding the re-excisions.
-
•
The cosmetic outcome can be obtained minimizing the resection of healthy-tissue.
1. Background
The rate of non-palpable breast lesions detected during screening programmes is increasing [1] worldwide. These patients are often eligible for breast-conserving surgery (BCS) since it has shown a disease-free survival rate equivalent to those of mastectomy, offering the advantages of cosmetic outcome preservation, a better quality of life and a decrease in psychological morbidities [2]. These important aspects, along with the general goal of reducing the extent of surgical treatment, have increased the need for an effective pre-surgery lesion localization. A precise preoperative localization technique for the detection of infiltrating small lesions and ductal carcinoma in situ (DCIS) [3] by improved imaging technologies as in the case of micro-calcification or distortions, is even more important. The procedure should be comfortable enough for the radiologist, who is responsible for the localization of the right lesions through the effective placement of the device, avoiding at the same time any further displacement. Subsequently, the surgeon aims at resecting the non-palpable lesions ensuring clear margins, while avoiding re-excisions. To minimize the resection of healthy-tissue preserving the cosmetic outcome is a further, not secondary, objective. All these factors coexist in a context of a better patient care, including patients' satisfaction and the optimization of centre resources. For these purposes, different techniques for the pre-surgery localization of breast lesions have evolved over time, since the adoption in the late ’70s [4] of the currently widely used wire guided localization (WGL) technique, where the tip of a flexible wire is anchored to the lesion. Over time, though, this procedure has faced several limitations [5]: (i) wire dislodging [11], migration [6] or wire fracture; (ii) higher patient discomfort and (iii) logistic difficulties, as the wire is to be placed no more than one day before surgery. Moreover, poor cosmetic outcomes have been described [7,8] and high rates of a non-radical excision of the lesion due to wire dislodging and poor localization have been reported [9]. With the same principle of tracing the path from the skin to the lesion, avoiding the use of the wire, a sterile charcoal powder diluted with saline solution was injected near the lesion [10,11] creating a trail (with a continuous injection during the needle release) to guides the surgeon during the following operation. The main advantages, beyond the dislodging, are logistics, patient comfort and costs.
Radioactive guided localization techniques (RGLT), consisting of a radioactive substance to be placed into abnormal breast tissue and localized during the surgery using a handheld gamma probe, represented the first reliable non-wired (NW) alternative, overcoming the aforementioned issues. More specifically a titanium capsule containing radioactive Iodine-125, named radioactive seed localization (RSL) and a 99mTc-radiolabelled albumin-based colloid, named radio-guided occult lesion localization (ROLL) were introduced in the early 2000s.
Both the RGLT have been reported as safe and reliable, offering several clinical and organizational advantages [5,12,13], including a lower re-excision rate [14], with the potential to decouple the radiology and surgery schedules, and improve patients’ satisfaction [15]. The radiologist is facilitated by the possibility to choose any skin entry site for the accurate placement of the device [16], and the surgeon has more flexibility in choosing the incision location, improving cosmetic outcomes [17].
A recent meta-analysis [18] on preoperative localization techniques in breast conservative surgery, confirms the superiority of RGLT (with a slightly superiority of RSL) when compared to wire, representing a potential valid alternative. However, the most commonly cited drawback for these techniques is the presence of radioactivity in the implanted seed/colloid, with important restrictions for patients and extensive multidisciplinary coordination and regulatory compliance [12]. The use of a radiation-free titanium clip embedded with a collagen plug positioned after biopsy and visible on the ultrasound has been also proposed [19]. This and other available techniques on preoperative localization have been exhaustively described by Morerira et al. [18] and have therefore not been taken into consideration in our analysis.
Other simple approaches for the localization of non-palpable lesions, that does not require specific technology, utilize sonographic or mammographic images for the visualization of the tumors at their largest diameter. This allows to achieve the optimal correspondence between the lesion and the skin where tumor's projection is pointed [20]. Differently, where surgeons have specific competences on ultrasound, these can intraoperatively locate the lesion, measures its diameter and distance from surrounding hallmarks and directly proceed with the surgery.
In our analysis, we focus on the classical localization exploring the latest technological evolution that has overcome the radioactivity limitation by introducing small magnetic seeds (Magseed, MaMaLoc and MOLLI) or electromagnetic-radiofrequency (RFID) tags (SAVI SCOUT and LOCalizer). These small devices, preloaded into a 12–18 gauge needle introducers, are deployed into the lesion by radiologists up to more of than 30 days before surgery. A specific probe, connected to a console that emits audio and visual feedback in proportion to the localizer distance, is used in the operating room.
Even with functional and some technical differences already well described [12,21], these non-wired non-ionizing (NWNI) devices may represent the optimal choice for the preoperative non-palpable breast lesion localization. Studies aimed to evaluate safety, efficacy and effectiveness have been carried out on small numbers of patients and not randomized. Therefore, we aimed to review all the available trials describing the use of NWNI localization techniques to provide more precise estimates for main outcomes: device placement effectiveness, positive margins, clear margins and re-excision rates.
2. Materials and methods
2.1. Data sources and search strategy
The review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [22], as illustrated in Appendix A. A summary of Population, Intervention or exposure, Comparison, Outcomes, and Study design (PICOS) parameters used to describe inclusion and exclusion criteria for this literature review are reported in Table 1.
Table 1.
Summary of Population, Intervention or exposure, Comparison, Outcomes, and Study design (PICOS).
| Parameter | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | Women with non-palpable breast cancer | Women with other cancer types |
| Intervention or exposure | Localization of cancer lesion with magnetic seeds or radiofrequency tags | |
| Comparison | None or with wired guided localization | |
| Outcomes | Seeds' successful placement and localization rates; reflector distance from skin and from target; number of days before surgery; clear, close and positive margins rate; re-excision rate; malignant invasive cancer and DCIS rates | |
| Study design | Prospective clinical trials and retrospective observational studies | None |
We conducted a literature review in Medline, Scopus and Embase based on the following terms: “breast, localization, lesion, non-palpable, preoperative, guidance, savi-scout, LOCalizer, magseed, mamaloc, molli, magnetic, radiofrequency, wireless, non-wired, non-radioactive” (main search query in Appendix B).
2.2. Eligibility criteria and data sources
Prospective clinical trials and retrospective studies were included; conference abstracts were not considered. In the full text, the following outcomes have to be included: the total number of patients undergoing lesion localization with non-ionizing technologies, successful placement/localization of the devices, and their successful identification/retrieval. Furthermore, information on the distance of the reflector from skin and target, and the number of days prior to surgery was collected. Data regarding clear, close and positive margins, re-excision rates, the presence of malignant invasive cancer and DCIS were also extracted and analyzed when available.
2.3. Data sources
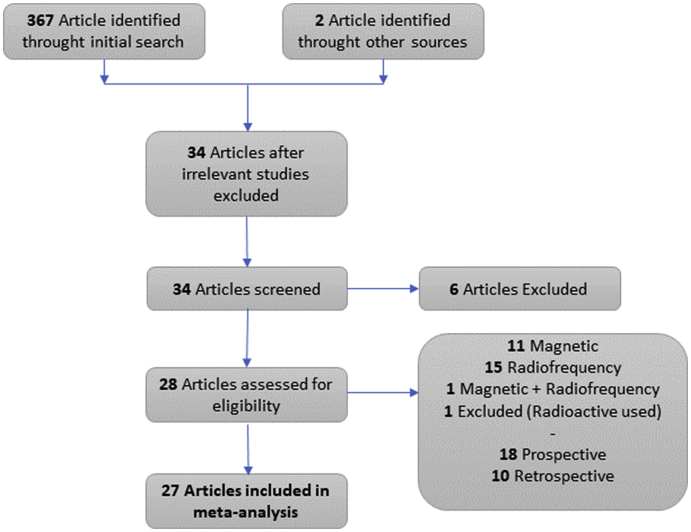
Fig. 1 presents the PRISMA flowchart [22]. The studies were eligible if they had been published in the English language up to February 2021. Besides, additional sources were sought in the references of all retrieved eligible papers.
Fig. 1.
Flowchart of literature search based on PRISMA example.
2.4. Data collection and management
Two independent reviewers (FG and RIC) screened the title/abstract of the selected records. Then, full texts were retrieved for further assessment, and each assessor independently extracted information from the eligible studies. Discrepancies were solved through discussion between the two reviewers in each phase of the review; a third author was consulted when consensus was not achieved. All the information extracted from the eligible studies was collected in a standard Microsoft Excel© sheet.
2.5. Outcomes
Primary outcomes considered in this meta-analysis were procedure's success and NWNI's localization rates, surgical margins status (clear, close, positive) and re-excision rate. As secondary outcomes the pooled rate of invasive breast cancer (IBC) and DCIS, the mean NWNI devices' distance from target and from patients' skin and days of placement before surgery were considered. All outcomes were explored also comparing NWNI and WGL, in addition to specimen's volume and weight.
2.6. Bias assessment
It was found that conventional funnel plots (plots of the log of effect measures versus standard error) are inaccurate in the analysis of publication bias of studies reporting proportional outcomes because they produce spurious asymmetry in the plot, even when publication bias does not exist [23]. Because of the diversity study designs adopted, unequal and small sample sizes and the limited application of publication bias assessment in studies of the prevalence, the risk of bias of individual studies has been assessed following the ROBINS-I tool [24].
2.7. Statistical analysis
All parameters were tabulated for the meta-analysis. For continuous outcomes, the effect was measured using the difference in means, as outcome measurements in all studies are made on the same scale [25]. For a statistical analysis, the continuous outcomes were described in averages corrected for sample size, while the binomial outcomes were described using the weighted average proportion [26], which is an average of the results (i.e., proportions) of all the considered studies weighted by the inverse of their sampling variances using either the fixed-effects or random-effects model [27]. Results were presented from the random-effects model. The heterogeneity between study-specific estimates was tested using Chi-square statistics and measured with the I2 statistics (a measure of the percentage variation across studies caused by heterogeneity) [28]. The comparison of specimen's volume and weight between NWNI and WGL techniques was presented using standardized mean difference between the two localization methods.
The analyses were performed using R 3.6.1 [29] with metafor [30] and meta [31] packages.
3. Results
3.1. Study selection
In the initial research, 367 studies were identified. After the exclusion of several studies, as illustrated by PRISMA flow chart in Figs. 1, 27 studies were included in the final analysis (Table 2). This meta-analysis included articles published between 2016 and 2021. The study design of the included studies was prevalently prospective (18, 66,7%), involving from 4 to 320 patients each. The devices used in the studies were: SAVI SCOUT (9, 33.3%) [[32], [33], [34], [35], [36], [37], [38], [39], [40], [41]], Magseed (10, 37%) [38,[42], [43], [44], [45], [46], [47], [48], [49], [50]], Localizer (5, 18.5%) [38,40,[51], [52], [53], [54], [55]] and MaMaLoc (1, 3.7%) [56]. One study reported results about the use of both Magseed and LOCalizer [38] and in one both SAVI SCOUT and LOCalizer were used [40]. Among all these studies, 8 of them compared the use of NWNI and wire localization techniques [35,37,[39], [40], [41],48,49,53]. The MOLLI have been only evaluated for safety and clinical feasibility [57] and is not included in our analysis. These studies included 2001 subjects with non-palpable breast lesions, for a total of 2103 devices used. The main reason for the lesion localization was for breast-conserving surgery (1846 patients, 92%) and for excisional biopsy (152 patients, 8%). 60.5% of the procedures were implemented under sonographic guidance. We have not deepened the technological aspects of the devices being already well described in literature [12,21,58].
Table 2.
Study characteristics.
| Study | N. of patients | Age of patients [median (IQR) or mean (SD) | N. of seeds | Type of localization | Time of seeds' placement (before surgery) | Study design | Type of study | Single arm/comparison group | Device | Main outcomes | Margin definition |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cox, 2016 [32] | 154 | 58.8 (29–89) | 154 | Excisional biopsy, 40; Lumpectomy, 113 |
0–7 days | Prospective | Multicenter | Single arm | SAVI SCOUT | Rates of successful reflector placement, localization, and retrieval, percent of clear margins, percent of cases requiring re-excision, physician experience compared with WL, patient comfort, and overall experience | <1 mm (close margin) |
| Mango, 2016 [33] | 13 | 52.5 (NA) | 15 | Excisional biopsy, 5; Lumpectomy, 10 |
0–6 days | Retrospective | Monocentric | Single arm | SAVI SCOUT | Rates of target and reflector removal, pathologic results, complications and RE-Rs | NA |
| Mango, 2017 [34] | 100 | 56.4 (24–82) | 123 | NA | 0–8 days | Retrospective | Monocentric | Single arm | SAVI SCOUT | Rates of reflector placement, localization, and removal, target excision and RE-Rs | Ink on tumor (positive margin), <1 mm (close margin) |
| Patel, 2017 [35] | 42 | 62.5 (±11.3) | 42 | Lumpectomy, 42 | 1–10 days | Retrospective | Monocentric | Comparison group (wire localization) | SAVI SCOUT | Final surgical pathology, histologic type, margin status, and RE-Rs | Ink on tumor (positive margin), ≤1 mm (close margin) |
| Schermers, 2017 [56] | 15 | NA | 15 | All lumpectomies | 0–30 days | Prospective | Monocentric | Single arm | MaMaLoc | Identification rate, tumor features, physician experience | NA |
| Falcon, 2018 [36] | 129 | NA | 152 | All lumpectomies | 0–27 days | Retrospective | Monocentric | Single arm | SAVI SCOUT | Rates of reflector placement, localization, and removal, | NA |
| Harvey, 2018 [43] | 28 | 54 (37–75) | 29 | All total mastectomies | 2–30 days | Prospective | Multicenter | Single arm | Magseed | Distribution of seed migration, accuracy of initial placement, relationship between depth of seed placement and ease of transcutaneous detection, seed integrity, safety and tolerability, total mastectomy weight and relationship between clinical characteristics and movement of the seed |
NA |
| Lamb, 2018 [44] | 188 | 59 (22–89) | 213 | NA | NA | Retrospective | Monocentric | Single arm | Magseed | Number of localization markers placed, indications for marker placement, lesion type, imaging guidance used for marker placement, post-procedure mammographic imaging and reports, surgical reports, and surgical margin status |
NA |
| Pohlodek, 2018 [45] | 10 | 48.7 (NA) | 10 | All lumpectomies | NA | Prospective | Monocentric | Single arm | Magseed | Rates of reflector localization, and removal, surgical margin status | NA |
| Price, 2018 [42] | 64 | 58 (25–86) | 73 | Biopsy, 12; Lumpectomy, 61 |
0–40 days | Prospective | Monocentric | Single arm | Magseed | Localization features, surgical features, and pathologic results | NA |
| DiNome, 2019 [51] | 50 | 59.2 (NA) | 50 | All lumpectomies | 0–14 days | Prospective | Monocentric | Single arm | LOCalizer | Rates of reflector placement, localization, and removal, patient, radiologist, and surgeon experience, surgical margin status and RE-R, volume of tissue removed, days prior to surgery of Tag insertion, Tag migration |
NA |
| Malter, 2019 [52] | 4 | 40.7 (±10.3) | 4 | All lumpectomies | NA | Prospective | Monocentric | Single arm | LOCalizer | Rates of reflector placement, localization, and removal | NA |
| McGugin, 2019 [53] | 147 | 59.5 (±14.0) | 147 | Biopsy, 53; Lumpectomy, 94 |
1–22 days | Retrospective | Multicenter | Comparison group (wire localization) | LOCalizer | Median specimen volume, operative time, and RE-R | NA |
| Pohlodek, 2019 [46] | 38 | 56.5 (NA) | 41 | All lumpectomies | NA | Prospective | Monocentric | Single arm | Magseed | Rates of reflector placement, localization, and removal, surgical margin status and RE-R | No ink on tumor + tumor free margins < 2 for DCIS (negative margin) |
| Srour, 2019 [37] | 108 | 66 (25–89) | 108 | Biopsy, 30; Partial mastectomy, 78 |
0–18 days | Prospective | Multicenter | Comparison groups (wire and radioactive seed localization) | SAVI SCOUT | Delay in operating room start times and total perioperative times in both the hospital and ambulatory setting, localization time, explant of localization device, positive margins, volume of tissue excised, and 30-day complications | NA |
| Thekkinkattil, 2019 [47] | 137 | 60 (28–81) | 139 | Diagnostic biopsy, 16; Brest-conservation surgery, 121 |
0–30 days | Prospective | Multicenter | Single arm | Magseed | Weight of resection specimen, type of surgery, mode of insertion, time of insertion, histology, RE-R, and details any perioperative complications | <1 mm (positive margin) |
| Zacharioudakis, 2019 [48] | 104 | 60.9 (NA) | 104 | All lumpectomies | NA | Prospective | Multicenter | Comparison groups (wire localization) | Magseed | Rates of reflector placement, successful identification and excision of the lesion, RE-R, specimenweight, specimen size, and lesion to specimen size ratio |
<1 mm (positive margin) |
| Cullinane, 2020 | 69 | 56 (50–65) | 69 | Diagnostic biopsy, 6; Brest-conservation surgery, 63 | NA | Prospective | Monocentric | Single arm | LOCalizer | Margin positivity and RE-R, data on patients' age, subtype, grade, specimen weight and size and complication rate | Ink on tumor + tumor free margins < 2 mm for DCIS (positive margin) |
| Fung, 2020 | 21 | 60.0 (±11.6) | 22 | Diagnostic intent, 16; Therapeutic excision, 3 | 6–56 days | Retrospective | Monocentric | Single arm | Magseed | Rates of reflector placement, localization, and removal, RE-R, complications | <1 mm (positive margin) |
| Lee, 2020 | 21 | 63.4 (±12.1) | 21 | All lumpectomies | NA | Retrospective | Monocentric | Comparison groups (LOCalizer and wire localization) | SAVI SCOUT | Rates of scheduled first starts, positive margins and RE-R, operative duration, pathologic lumpectomy volumes, tumor histology and T stage | No ink on tumor + tumor free margins < 2 for DCIS (negative margin) |
| Lee, 2020 | 33 | 61.3 (±11.4) | 33 | Comparison groups (SAVI SCOUT and wire localization) | LOCalizer | ||||||
| Micha, 2020 | 128 | 137 | All lumpectomies | NA | Prospective | Multicenter | Comparison groups (wire localization) | Magseed | Patient and clinician satisfaction, demographic data, specimen weight, tumour size, positive margin rates, and RE-R | <1 mm (positive margin) | |
| Pieszko, 2020 | 10 | 60.6 (42–79) | 10 | All lumpectomies | NA | Prospective | Monocentric | Single arm | Magseed | Rates of reflector placement, localization, and removal, surgical margin status and RE-R, weight of resected specimens, migration rates of the marker | NA |
| Srour, 2020 | 16 | 60.38 (±14.73) | 16 | Biopsy, 3; Partial mastectomy, 13 |
NA | Prospective | Monocentric | Comparison groups (radioactive seed and wire localization) | SAVI SCOUT | Delay in operating room start times, total perioperative times, and 30-day complications, demographics, number of lesions, use of bracketing, operating room setting, successful explant of localization device and target, operating room scheduled time, margin status, pathology, volume of tissue excised and RE-R | |
| Tayeh, 2020 | 20 | 50.8 (27–76) | 23 | Diagnostic intent, 4; Therapeutic excision, 19 | 0–8 | Prospective | Monocentric | Single arm | SAVI SCOUT | Rates of reflector placement, localization, and removal, surgical margin status and RE-R, duration of localization/insertion; duration of identification and retrieval; weight of the resected specimen; migration rate; status of the radial surgical margins and RE-R, feedback of patients, radiologists, and surgeons | final radial margin >2 mm for DCIS and > 1 mm for invasive cancer (clear margin) |
| Tayeh, 2020 | 6 | 56 (26–77) | 6 | NA | 0–30 | Prospective | Monocentric | Comparison groups (Magseed) | LOCalizer | Rates of reflector placement, localization, and removal, surgical margin status and RE-R, specimen weight, migration rate, and the perspectives of patients, radiologists and the surgeon | ≥2 mm for DCIS and ≥1 mm for other lesions (clear margin) |
| Tayeh, 2020 | 16 | 16 | Comparison groups (LOCalizer) | Magseed | |||||||
| Tingen, 2020 | 320 | 61.2 ± 12.1 | 320 | All lumpectomies | NA | Prospective | Monocentric | Comparison groups (wire localization) | SAVI SCOUT | Patient demographics, body mass index, method of localization, lesion type, complication, positive margin, and RE-R | Ink on tunor or < 1 mm (positive margin) |
| Wazir, 2020 [54] | 10 | 52.9 (40–68) | 11 | All lumpectomies | 0–7 days | Prospective | Monocentric | Single arm | LOCalizer | Rates of reflector placement, localization, and removal, surgical margin status and RE-R, weight of resected specimens, migration rates of the marker, acceptance by patients, radiologists and surgeons | NA |
Abbreviations: NA, not available.
3.2. Primary outcomes
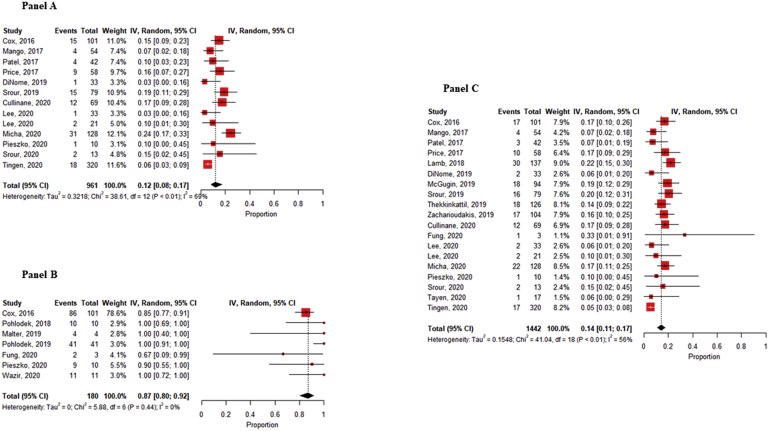
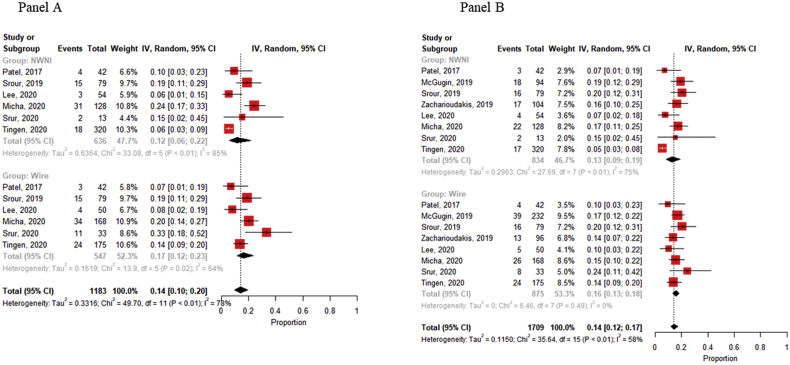
In all included studies, the outcomes “success of NWNI devices placement” and “number of NWNI devices localized” was defined. Overall, the rates of both the success of the procedure (in terms of proximity to the lesion) and the localization of the reflector were 98% (95% CI, 96–98% and 97–99%, respectively). The positive and clear margin rates (mainly defined as tumor cells ≤ 1 mm from the resection margin and tumour free-margin more than 1 mm, respectively) [59] were 12% (95% CI, 8–17%) and 87% (95% CI, 80–92%), with no observed heterogeneity for clear margin rate (Fig. 2 – Panel A & B). These outcomes were reported on 13 out of the 27 studies (totalizing 961 procedures) and 7/27 (180 procedures) respectively. Overall, the re-excision rate (19/27 1442 procedures), was 14% (95% CI, 11–17%) (Fig. 2 – Panel C). Comparing NWNI and wire localization techniques, it could be observed that positive margin rate (Fig. 3, Panel A) is lower for NWNI techniques (12%, 6–22% vs 17, 12–23%) and re-excision rate (Fig. 3, Panel B) is slightly higher using WGL (13%, 9–19% vs 16%, 13–18%), but both results are not statistically significant. Heterogeneity was observed for both outcomes (p < 0.01).
Fig. 2.
Panel A Pooled estimates of positive margins rates. - Panel B Pooled estimates of clear margins rates. - Panel C Pooled estimates of re-excision rates. Squares represent study-specific estimates (size of the square reflects the study-specific statistical weight); horizontal lines represent 95% CIs; diamonds represent the pooled estimate with corresponding 95% CI; p values are from testing for heterogeneity between study-specific estimates. Abbreviation: CI, confidence interval.
Fig. 3.
Panel A Pooled estimates of positive margins rates among NWNI and WGL techniques. Panel B Pooled estimates of re-excision rates among NWNI and WGL techniques, Squares represent study-specific estimates (size of the square reflects the study-specific statistical weight); horizontal lines represent 95% CIs; diamonds represent the pooled estimate with corresponding 95% CI; p values are from testing for heterogeneity between study-specific estimates. Abbreviation: CI, confidence interval; NWNI, non-wired non-ionizing.
No serious clinical complications were reported. In one study, two patients were converted to the wire technique because the devices were deployed far from the target. Further migrations have been associated with the accordion effect that cause the migration of the marker on release of the breast from compression during the stereotactic guidance [44,60].
Hematoma has been reported (up to 19%): after biopsy [34,38] (leading also to a possible difficulty in obtaining the signal from the device [34]), following the localization [48], and post operatively [33,39,49,55].
3.3. Secondary outcomes
The pooled rates of invasive breast cancer (IBC) and DCIS, calculated among 17 studies, were 68% (95% CI, 58–76%) and 25% (95% CI, 18–33%) respectively. Heterogeneity among studies was observed in both cases (p < 0.01). The mean distance of the NWNI from the target (cancer lesion) was 2.46 mm (95% CI, 0.9–4.03 mm), and from the patients’ skin 2.32 cm (95% CI, 1.71–2.94 cm). Overall, the devices were placed on average 5.19 days before surgery (95% CI, 3.28–7.11 days). with a great heterogeneity among studies (p < 0.01) [Data not shown].
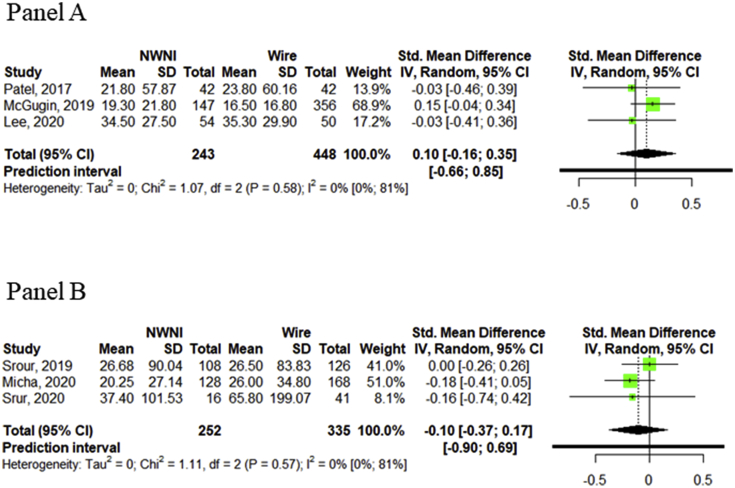
Comparing secondary outcomes between NWNI and WGL, results were similar to those described so far. Only 3 studies out of 8 reported data about specimen's volume [35,40,53] and weight [37,41,49]. Observing differences between the two localization techniques, specimen's volume (Fig. 4, Panel A) was slightly higher if the lesion were localized with wire technique (SMD 0.10 cm3, 95% CI, −0.16-0.35 cm3) and the weight (Fig. 4, Panel B) was lower for NWNI localizations (−0.10 gr, −0.37-0.17 gr). No heterogeneity was observed in both cases.
Fig. 4.
Panel A Comparison of specimen's volume between NWNI and WGL techniques. Panel B Comparison of specimen's weight between NWNI and WGL techniques. Squares represent standardized mean difference (size of the square reflects the study-specific statistical weight); horizontal lines represent 95% CIs; diamonds represent the pooled standardized mean difference with corresponding 95% CI; p values are from testing for heterogeneity between study-specific estimates. Abbreviation: CI, confidence interval; NWNI, non-wired non-ionizing; Std, standardized.
3.4. Bias assessment
Although studies included in the analysis were heterogenous in terms of design and number of patients enrolled, all of them were classified as “low risk” of bias through the ROBINS-I tool's own domains that included bias due to confounding, participants selection, classification of interventions, deviations from intended intervention, missing data, outcomes considered and reported results.
4. Discussion
The purpose of the present meta-analysis was to evaluate the clinical outcomes of all studies describing the preoperative use of NWNI techniques in patients with non-palpable breast lesions. Although these techniques are being increasingly used as they allow to overcome some issues that arise using the standard WGL techniques, studies on this regard are often on small numbers of patients and not randomized. Therefore, this study aims to provide overall estimates of the main and most important outcomes reported in the literature.
Most of the studies in this meta-analysis are prospective (66.7%) and involve from 4 to 320 patients each. Ten studies used the Magseed system (Endomag limited, Cambridge, UK), and another nine studies used the SAVI Scout system (Cianna Medical, Aliso Viejo, CA, USA). The LOCalizer™ system (Hologic Inc., Santa Clara, CA, USA), the most recent technique into the wireless occult breast lesion localization ecosystem was used in five studies; only one study used the Magnetic Marker Localization (MaMaLoc) (Sirius Medical, Eindhoven, Netherlands). SAVI SCOUT has been used also for the resection of non-palpable soft tissue tumors [61].
All studies reported the success of the procedure and a nearly 100% reflector localization rate. Therefore, it can be argued that this technique is consolidated and does not imply particular operational difficulties in positioning and locating the devices. This rate is similar to that reported for radioactive seed/colloid localization, without the logistical challenges and risks related to this technique (concerning storage, handling, and retrieving radioactive seeds). Among the 27 studies analyzed, a post operational pneumothorax [42] was recorded while no significant technical complications emerged. Operatively, the difficulties were mainly related to post biopsy hematoma. Caution is warranted in patients with surgical site occurrences, but this independently on the localization technique.
The lack of an external connection, the most noticeable characteristics of the NWNI devices, preserves from infections and possible displacement. On WGL, these complications can occur during patient transport or intraoperatively [62] with severe migrations involving the intrathoracic space up to the pleural cavity. Pericardium and pulmonary hilum were rarely reached [63,64], but with a high risk for serious complications. A vasovagal reaction was also reported on 7% of the procedures [65]. If some dislocations/migrations are also possible with NWNI techniques, these do not cause such harm for patients.
Considering the primary surgical outcomes, evidences on clear and positive margin rates and re-excision rate when compared to WGL are not supported by randomised trials. The studies [35,37,[39], [40], [41],48,49,53] that have directly compared NWNI with WGL show, in our pooled analysis, a lower positive margin rate for NWNI techniques and a higher re-excision rate for WGL, but without achieving a significant difference (according with the results of the single studies).
Important deviations have been observed in subgroups of patients [38] that should be carefully accounted for in future studies.
Given the limited evidences available on a direct comparison between the techniques, we look at the results obtained from studies on WGL: a clear margins rate of 70.8%–87.4% [17] is lower or in line with that obtained in our pooled results of 87%. Speculating, these data suggest a good ability of NWNI technique to correctly identify the lesions. Moreover, we found an overall margin positivity rate of 12% (95% CI, 8–17%) that corresponds to the findings of a large randomized trial [66] (when DCIS are excluded) but it is lower than that reported in large series studies (16.4%–20.8%) [3,67,68]. This latter difference may be explained with a lower incidence of DCIS, an independent risk factor for positive margins [68] and re-operation (OR 3.82, 95% CI, 3.19–4.58). Reasons for margins involvements have been recently proposed by Micha et al. [49] and includes: pre-operative underestimated size of the lesion, inaccuracy on the localization, too small specimen removal and lesion not central with the specimen.
For what concern the pooled re-excision rate of our analysis, it is lower if compared to that reported for studies on WGL (14% vs 21%), and in line with those reported after the 2014 consensus [59]. Again, these comparisons with WGL studies are not sufficient to support the superiority of NWNI technology and serve primarily as input for the design of randomized trials aimed at further investigating the outcome of interest.
Among the clinical and procedural variables, including SS-ASTRO guidelines, cavity shaves, intraoperative pathology and several others [69], the lesion localization of breast cancer has been strongly recommended by a conference consensus aimed to reduce the overall reoperation rate (RR). The results are obtained by considering systematic reviews on RSL vs WL [9,70,71] and ultrasound techniques. Our meta-analysis on NWNI techniques is a first attempt to discover their clinical effectiveness on RR and stimulate further in-depth analysis. Several initiatives, including a toolbox, has recently taken to reduce its occurrence [[72], [73], [74], [75]].
The burden of a reoperation involves a return to the operating room, prolonged recovery and a trauma for patients and family, in addition to an increase in poor cosmetic outcome and additional costs. Currently, there is no consensus on how to measure the cosmetic outcome, as there are several issues to consider. Nevertheless, accepted risk factors for poor cosmetic are represented by the shape and size of the tumour, its location, the tumour-breast ratio, postoperative radiation and the total volume removed [76].
The wire entry point is somewhat constrained for radiologists, especially for DCIS under sonographic guidance, and may dictate the surgery incision path. The ability to choose the incision site closest to the lesion, as in the case of the new technologies introduced (including NWNI), minimizes non-target tissue removal [16]. Our pooled analysis did not find significant differences in terms of specimen's volume or weight between NWNI and wire techniques. The data in this regard are too weak for further considerations. In an unpowered randomized study, the overall result was good or excellent, but no difference was found comparing localization techniques [76], as highlighted by another study using the Harvard scale for a retrospective evaluation [77].
The pooled analysis shows that all NWNI devices were implanted several days before surgery (mean 5.19, 95% CI, 3.28–7.11), even if with different extents between studies. This is in line with the information reported on all these techniques, which can be deployed at any time prior to surgery [54]. It is an important aspect from the point of view of the organization and allocation of resources, as well as of patients' quality of life. The wire should be placed immediately before surgery, rising possible logistical problems between the surgeon and the radiology schedule that can cause delays in surgical start time. In fact, preoperative wires are usually placed on the same day of the breast surgery and in the same building where the surgery is scheduled. Furthermore, due to the nature of the WGL techniques, it requires the patients' greater compliance, as the wire must remain in position between the time of deployment and surgical excision [17], and could importantly affect the patients’ quality of life.
No studies included in this meta-analysis have investigated the cost-effectiveness of these strategies. To our best knowledge, only one study has evaluated the expected budget impact of adopting magnetic seeds localization compared to the standard of care (WGL and RSL) [78], showing that magnetic seeds localization could be a new cost-efficient localization technology in guiding the resections of non-palpable breast cancer tumors.
NWNI techniques need an early capital expenditure for the intraoperative detection equipment to be used and most of the cost savings are indirect: the most noticeable, if compared with RSL, is the unnecessary involvement of nuclear medicine personnel and resources. All the cited techniques have direct and indirect costs that shall be carefully evaluated for their cost assessment, as done in several papers [[79], [80], [81], [82], [83], [84]]. It is noteworthy that all cost-savings (both direct and indirect) can vary from institution to institution depending on several logistic factors, such as the location of the theatre and admission wards, the radiologists’ workloads, the structure of the clinics, and the cancer workload.
4.1. Strengths and limitations
This is the first study that provides pooled estimates of the main technical and surgical outcomes among subjects undergoing a preoperative localization of breast cancer lesions with NWNI devices. As there are few studies that have reported these outcomes compared with other localization strategies, it would be important to address several issues that allow to evaluate the real effectiveness of these methodologies with respect to gold standard ones. Randomized clinical trials are necessary to confirm our findings and observations, as well as to define the role of these new techniques in daily clinical practice. Moreover, our data can be useful for the design and the appropriate sample size calculation of future studies.
This analysis has several limitations. First of all, the size of the tumour was not sufficiently taken into account since it was poorly reported, including the specimen weight/tumour size, and it was difficult to accurately measure the dimensions of a tissue specimen ex vivo, even if its dimension plays an important role on the tumour excision [85]. The patients undergoing excisional biopsy for benign lesions should not be included in calculations of margins and re-excision rates. Then, the lack of randomized studies allows for an only partial evaluation of the effectiveness of clinical outcomes, themselves often penalized by the lack of consensus, associated with these techniques. Moreover, conference abstracts were not considered in this review and meta-analysis, and it could have introduced some bias in the analysis. Then, since the groups related to the different devices used are numerically very different (and therefore unbalanced), and there is no homogeneity in reporting outcomes, subgroup analyses were not conducted. Lastly, no study considered for this meta-analysis reported data on cost-effectiveness of NWNI localization strategies. Therefore, further studies are needed in order to address all these issues and provide a complete picture of the real effectiveness of these new promising techniques.
4.2. Conclusions
In conclusion, preoperative NWNI techniques for the localization of non-palpable breast lesions are promising techniques in obtaining clear margins and on the re-operation rates. These new strategies are comparable to WGL and RSL in terms of the success of the procedure, the reflector localization rates and the margin positivity rates. NWNI devices can be placed several days before surgery without risks associated with WGL and without limitations related to RSL. Moreover, it allows both the radiologist and the surgeon to work in total decision-making autonomy and to avoid the onset of management issues related to the organization and allocation of resources. Finally, these localization strategies achieve the primary goal of BCS, that is to remove the lesion with negative margins, with a particular attention in preserving the cosmetic aspect, leading to an improved quality of life in this specific population. However, more robust randomized trials and consensus assessments are necessary to further confirm the present findings and allow for improvements on patient outcomes.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of supporting data
Not applicable.
Declaration of competing interest
All authors report no conflict of interest to declare.
Acknowledgements
Fundings: No sources of funding are declared for this manuscript.
Appendix A.
Appendix B.
Main search query
(Breast lesion∗?[Title] OR Breast cancer[Title] or Nonradioactive[Title]) AND (loca∗[Title]) AND (magnetic[Title/Abstract] OR radiofreq∗[Title/Abstract] OR magseed∗[Title/Abstract] OR LOCalizer[Title/Abstract] OR SAVI[Title/Abstract] OR mamaloc[Title/Abstract] OR molli[Title/Abstract]) NOT (ROLL[Title/Abstract] OR MRI[Title/Abstract] OR MR[Title/Abstract]).
References
- 1.Bick U., Trimboli R.M., Athanasiou A., Balleyguier C., Baltzer P.A.T., Bernathova M. Image-guided breast biopsy and localisation: recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights Imaging. 2020;11 doi: 10.1186/s13244-019-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Veronesi U., Cascinelli N., Mariani L., Greco M., Saccozzi R., Luini A. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 3.Langhans L., Jensen M.-B., Talman M.-L.M., Vejborg I., Kroman N., Tvedskov T.F. Reoperation rates in ductal carcinoma in situ vs invasive breast cancer after wire-guided breast-conserving surgery. JAMA Surg. 2017;152:378. doi: 10.1001/jamasurg.2016.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall F., Frank H. Preoperative localization of nonpalpable breast lesions. Am J Roentgenol. 1979;132:101–105. doi: 10.2214/ajr.132.1.101. [DOI] [PubMed] [Google Scholar]
- 5.Chan B.K., Wiseberg-Firtell J.A., Jois R.H., Jensen K., Audisio R.A. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD009206.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachir N., Lemaitre J., Lardinois I. Intrathoracic hooked wire migration managed by minimally invasive thoracoscopic surgery. Acta Chir Belg. 2020;120:186–189. doi: 10.1080/00015458.2018.1523298. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed M., van Hemelrijck M., Douek M. Systematic review of radioguided versus wire-guided localization in the treatment of non-palpable breast cancers. Breast Canc Res Treat. 2013;140:241–252. doi: 10.1007/s10549-013-2547-5. [DOI] [PubMed] [Google Scholar]
- 8.Dua S.M., Gray R.J., Keshtgar M. Strategies for localisation of impalpable breast lesions. Breast. 2011;20:246–253. doi: 10.1016/j.breast.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Pouw B, de Wit-van der Veen L.J., Stokkel M.P.M., Loo C.E., Vrancken Peeters M.J., Valdés Olmos R.A. Heading toward radioactive seed localization in non-palpable breast cancer surgery? A meta-analysis. J Surg Oncol. 2015;111:185–191. doi: 10.1002/jso.23785. [DOI] [PubMed] [Google Scholar]
- 10.Svane G. A stereotaxic technique for preoperative marking of non-palpable breast lesions. Acta Radiol Diagn. 1983;24:145–151. doi: 10.1177/028418518302400207. [DOI] [PubMed] [Google Scholar]
- 11.Arman A., Kilicoglu G., Guner H.H., Celik L. Marking of nonpalpable breast lesions using a custom carbon suspension. Acta Radiol Stockh Swed 1987. 2001;42:599–601. doi: 10.1080/028418501127347269. [DOI] [PubMed] [Google Scholar]
- 12.Mayo R.C., Kalambo M.J., Parikh J.R. Preoperative localization of breast lesions: current techniques. Clin Imag. 2019;56:1–8. doi: 10.1016/j.clinimag.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Niinikoski L., Hukkinen K., Leidenius M.H.K., Vaara P., Voynov A., Heikkilä P. Resection margins and local recurrences of impalpable breast cancer: comparison between radioguided occult lesion localization (ROLL) and radioactive seed localization (RSL) Breast. 2019;47:93–101. doi: 10.1016/j.breast.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Lovrics P.J., Goldsmith C.H., Hodgson N., McCready D., Gohla G., Boylan C. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann Surg Oncol. 2011;18:3407. doi: 10.1245/s10434-011-1699-y. [DOI] [PubMed] [Google Scholar]
- 15.Bloomquist E.V., Ajkay N., Patil S., Collett A.E., Frazier T.G., Barrio A.V. A randomized prospective comparison of patient-assessed satisfaction and clinical outcomes with radioactive seed localization versus wire localization. Breast J. 2016;22:151–157. doi: 10.1111/tbj.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray R.J., Salud C., Nguyen K., Dauway E., Friedland J., Berman C. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: radioactive seed versus wire localization. Ann Surg Oncol. 2001;8:711–715. doi: 10.1007/s10434-001-0711-3. [DOI] [PubMed] [Google Scholar]
- 17.Hayes M.K. Update on preoperative breast localization. Radiol Clin. 2017;55:591–603. doi: 10.1016/j.rcl.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Moreira I.C., Ventura S.R., Ramos I., Fougo J.L., Rodrigues P.P. Preoperative localisation techniques in breast conservative surgery: a systematic review and meta-analysis. Surg Oncol. 2020;35:351–373. doi: 10.1016/j.suronc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Corsi F., Bossi D., Combi F., Papadopoulou O., Amadori R., Regolo L. Radio-guided vs clip-guided localization of nonpalpable mass-like lesions of the breast from a screened population: a propensity score-matched study. J Surg Oncol. 2019;119:916–924. doi: 10.1002/jso.25409. [DOI] [PubMed] [Google Scholar]
- 20.Franceschini G., Mason E.J., Grippo C., D'Archi S., D'Angelo A., Scardina L. Image-guided localization techniques for surgical excision of non-palpable breast lesions: an overview of current literature and our experience with preoperative skin tattoo. J Personalized Med. 2021;11:99. doi: 10.3390/jpm11020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapoor M.M., Patel M.M., Scoggins M.E. The wire and beyond: recent advances in breast imaging preoperative needle localization. Radiographics. 2019;39:1886–1906. doi: 10.1148/rg.2019190041. [DOI] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunter J.P., Saratzis A., Sutton A.J., Boucher R.H., Sayers R.D., Bown M.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deeks J., Altman D.G. second ed. BMJ Publishing Group; London: 2001. Effect measures for meta-analysis of trials with binary outcomes. Syst. Rev. Healthc. Meta-Anal. Context. [Google Scholar]
- 26.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Publ Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang N. 2018. How to conduct a meta-analysis of proportions in R: a comprehensive tutorial. [DOI] [Google Scholar]
- 28.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team. R . R Foundation for Statistical Computing; 2020. A language and environment for statistical computing. [Google Scholar]
- 30.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36 doi: 10.18637/jss.v036.i03. [DOI] [Google Scholar]
- 31.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Base Ment Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cox C.E., Russell S., Prowler V., Carter E., Beard A., Mehindru A. A prospective, single arm, multi-site, clinical evaluation of a nonradioactive surgical guidance technology for the location of nonpalpable breast lesions during excision. Ann Surg Oncol. 2016;23:3168–3174. doi: 10.1245/s10434-016-5405-y. [DOI] [PubMed] [Google Scholar]
- 33.Mango V., Ha R., Gomberawalla A., Wynn R., Feldman S. Evaluation of the SAVI SCOUT surgical guidance system for localization and excision of nonpalpable breast lesions: a feasibility study. Am. J. Roentgenol. 2016;4 doi: 10.2214/AJR.15.15962. [DOI] [PubMed] [Google Scholar]
- 34.Mango V.L., Wynn R.T., Feldman S., Friedlander L., Desperito E., Patel S.N. Beyond wires and seeds: reflector-guided breast lesion localization and excision. Radiology. 2017;284:365–371. doi: 10.1148/radiol.2017161661. [DOI] [PubMed] [Google Scholar]
- 35.Patel S.N., Mango V.L., Jadeja P., Friedlander L., Desperito E., Wynn R. Reflector-guided breast tumor localization versus wire localization for lumpectomies: a comparison of surgical outcomes. Clin Imag. 2018;47:14–17. doi: 10.1016/j.clinimag.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 36.Falcon S., Weinfurtner R.J., Mooney B., Niell B.L. SAVI SCOUT® localization of breast lesions as a practical alternative to wires: outcomes and suggestions for trouble-shooting. Clin Imag. 2018;52:280–286. doi: 10.1016/j.clinimag.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 37.Srour M.K., Kim S., Amersi F., Giuliano A.E., Chung A. Comparison of wire localization, radioactive seed, and Savi scout ® radar for management of surgical breast disease. Breast J. 2020;26:406–413. doi: 10.1111/tbj.13499. [DOI] [PubMed] [Google Scholar]
- 38.Tayeh S., Gera R., Perry N., Michell M., Malhotra A., Mokbel K. The use of magnetic seeds and radiofrequency identifier tags in breast surgery for non-palpable lesions. Anticancer Res. 2020;40:315–321. doi: 10.21873/anticanres.13955. [DOI] [PubMed] [Google Scholar]
- 39.Tingen J.S., McKinley B.P., Rinkliff J.M., Cornett W.R., Lucas C. Savi scout radar localization versus wire localization for breast biopsy regarding positive margin, complication, and reoperation rates. Am Surg. 2020;86:1029–1031. doi: 10.1177/0003134820939903. [DOI] [PubMed] [Google Scholar]
- 40.Lee M.K., Sanaiha Y., Kusske A.M., Thompson C.K., Attai D.J., Baker J.L. A comparison of two non-radioactive alternatives to wire for the localization of non-palpable breast cancers. Breast Canc Res Treat. 2020;182:299–303. doi: 10.1007/s10549-020-05707-1. [DOI] [PubMed] [Google Scholar]
- 41.Srour M.K., Kim S., Amersi F., Giuliano A.E., Chung A. Comparison of multiple wire, radioactive seed, and savi Scout® radar localizations for management of surgical breast disease. Ann Surg Oncol. 2021;28:2212–2218. doi: 10.1245/s10434-020-09159-1. [DOI] [PubMed] [Google Scholar]
- 42.Price E.R., Khoury A.L., Esserman L.J., Joe B.N., Alvarado M.D. Initial clinical experience with an inducible magnetic seed system for preoperative breast lesion localization. Am J Roentgenol. 2018;210:913–917. doi: 10.2214/AJR.17.18345. [DOI] [PubMed] [Google Scholar]
- 43.Harvey J.R., Lim Y., Murphy J., Howe M., Morris J., Goyal A. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): a multi-centre, open-label cohort study. Breast Canc Res Treat. 2018;169:531–536. doi: 10.1007/s10549-018-4709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lamb L.R., Bahl M., Specht M.C., D'Alessandro H.A., Lehman C.D. Evaluation of a nonradioactive magnetic marker wireless localization program. Am J Roentgenol. 2018;211:940–945. doi: 10.2214/AJR.18.19637. [DOI] [PubMed] [Google Scholar]
- 45.Pohlodek K., Foltín M., Mečiarová I., Ondriaš F. Simultaneous use of magnetic method in localization of impalpable breast cancer and sentinel lymph nodes detection: initial experience. Nanomed. 2018;13:3075–3081. doi: 10.2217/nnm-2018-0220. [DOI] [PubMed] [Google Scholar]
- 46.Pohlodek K., Sečanský P., Haluzová I., Mečiarová I. Localization of impalpable breast lesions and detection of sentinel lymph nodes through magnetic methods. Eur J Radiol. 2019;120:108699. doi: 10.1016/j.ejrad.2019.108699. [DOI] [PubMed] [Google Scholar]
- 47.Thekkinkattil D., Kaushik M., Hoosein M.M., Al-Attar M., Pilgrim S., Gvaramadze A. A prospective, single-arm, multicentre clinical evaluation of a new localisation technique using non-radioactive Magseeds for surgery of clinically occult breast lesions. Clin Radiol. 2019;74:974. doi: 10.1016/j.crad.2019.08.018. e7-974.e11. [DOI] [PubMed] [Google Scholar]
- 48.Zacharioudakis K., Down S., Bholah Z., Lee S., Khan T., Maxwell A.J. Is the future magnetic? Magseed localisation for non palpable breast cancer. A multi-centre non randomised control study. Eur J Surg Oncol. 2019;45 doi: 10.1016/j.ejso.2019.06.035. 2016–21. [DOI] [PubMed] [Google Scholar]
- 49.Micha A.E., Sinnett V., Downey K., Allen S., Bishop B., Hector L.R. Patient and clinician satisfaction and clinical outcomes of Magseed compared with wire-guided localisation for impalpable breast lesions. Breast Cancer. 2021;28:196–205. doi: 10.1007/s12282-020-01149-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pieszko K., Wichtowski M., Cieciorowski M., Jamont R., Murawa D. Evaluation of the nonradioactive inducible magnetic seed system Magseed for preoperative localization of nonpalpable breast lesions – initial clinical experience. Współczesna Onkol. 2020;24:51–54. doi: 10.5114/wo.2020.93677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DiNome M.L., Kusske A.M., Attai D.J., Fischer C.P., Hoyt A.C. Microchipping the breast: an effective new technology for localizing non-palpable breast lesions for surgery. Breast Canc Res Treat. 2019;175:165–170. doi: 10.1007/s10549-019-05143-w. [DOI] [PubMed] [Google Scholar]
- 52.Malter W., Holtschmidt J., Thangarajah F., Mallmann P., Krug B., Warm M. First reported use of the faxitron LOCalizerTM radiofrequency identification (RFID) system in Europe - feasibility trial, surgical guide and review for non-palpable breast lesions. In Vivo. 2019;33:1559–1564. doi: 10.21873/invivo.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGugin C., Spivey T., Coopey S., Smith B., Kelly B., Gadd M. Radiofrequency identification tag localization is comparable to wire localization for non-palpable breast lesions. Breast Canc Res Treat. 2019;177:735–739. doi: 10.1007/s10549-019-05355-0. [DOI] [PubMed] [Google Scholar]
- 54.Wazir U., Tayeh S., Perry N., Michell M., Malhotra A., Mokbel K. Wireless breast localization using radio-frequency identification tags: the first reported European experience in breast cancer. In Vivo. 2020;34:233–238. doi: 10.21873/invivo.11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cullinane C.M., Byrne J., Akmenkalne L., O’ Leary D.P., Connors A.M., Corrigan M.A. The LOCalizer radiofrequency identification system: an effective new technology for localizing non-palpable breast lesions for surgery. Surg Innovat. 2020 doi: 10.1177/1553350620967853. 155335062096785. [DOI] [PubMed] [Google Scholar]
- 56.van der Hage J.A., Loo C.E., Vrancken Peeters M.T.F.D., Winter-Warnars H.A.O., Ruers TJM B. Schermers. Feasibility of magnetic marker localisation for non-palpable breast cancer. Breast. 2017;33:50–56. doi: 10.1016/j.breast.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Look Hong N., Wright F.C., Semple M., Nicolae A.M., Ravi A. Results of a phase I, non-randomized study evaluating a Magnetic Occult Lesion Localization Instrument (MOLLI) for excision of non-palpable breast lesions. Breast Canc Res Treat. 2020;179:671–676. doi: 10.1007/s10549-019-05499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheang E., Ha R., Thornton C.M., Mango V.L. Innovations in image-guided preoperative breast lesion localization. Br J Radiol. 2018:20170740. doi: 10.1259/bjr.20170740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moran M.S., Schnitt S.J., Giuliano A.E., Harris J.R., Khan S.A., Horton J. Society of surgical oncology–American society for radiation oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol. 2014;32:1507–1515. doi: 10.1200/JCO.2013.53.3935. [DOI] [PubMed] [Google Scholar]
- 60.Fung W., Wong T., Chau C., Yu E.L., Chan T., Chan R.L. Safety and efficacy of magnetic seed localisation of non-palpable breast lesions: pilot study in a Chinese population. Hong Kong Med J. 2020 doi: 10.12809/hkmj208559. [DOI] [PubMed] [Google Scholar]
- 61.Broman K.K., Joyce D., Binitie O., Letson G.D., Gonzalez R.J., Choi J. Intraoperative localization using an implanted radar reflector facilitates resection of non-palpable trunk and extremity sarcoma. Ann Surg Oncol. 2020 doi: 10.1245/s10434-020-09229-4. [DOI] [PubMed] [Google Scholar]
- 62.Homer M. Transection of the localization hooked wire during breast biopsy. Am J Roentgenol. 1983;141:929–930. doi: 10.2214/ajr.141.5.929. [DOI] [PubMed] [Google Scholar]
- 63.Azoury F., Sayad P., Rizk A. Thoracoscopic management of a pericardial migration of a breast biopsy localization wire. Ann Thorac Surg. 2009;87 doi: 10.1016/j.athoracsur.2008.10.069. 1937–9. [DOI] [PubMed] [Google Scholar]
- 64.Banitalebi H., Skaane P. Migration of the breast biopsy localization wire to the pulmonary hilus. Acta Radiol. 2005;46:28–31. doi: 10.1080/02841850510015956. [DOI] [PubMed] [Google Scholar]
- 65.Helvie M.A., Ikeda D.M., Adler D.D. Localization and needle aspiration of breast lesions: complications in 370 cases. Am J Roentgenol. 1991;157:711–714. doi: 10.2214/ajr.157.4.1892023. [DOI] [PubMed] [Google Scholar]
- 66.Langhans L., Tvedskov T.F., Klausen T.L., Jensen M.-B., Talman M.-L., Vejborg I. Radioactive seed localization or wire-guided localization of nonpalpable invasive and in situ breast cancer: a randomized, multicenter, open-label trial. Ann Surg. 2017;266:29–35. doi: 10.1097/SLA.0000000000002101. [DOI] [PubMed] [Google Scholar]
- 67.Haloua M.H., Volders J.H., Krekel N.M.A., Barbé E., Sietses C., Jóźwiak K. A nationwide pathology study on surgical margins and excision volumes after breast-conserving surgery: there is still much to be gained. Breast. 2016;25:14–21. doi: 10.1016/j.breast.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 68.Laws A., Brar M.S., Bouchard-Fortier A., Leong B., Quan M.L. Intraoperative margin assessment in wire-localized breast-conserving surgery for invasive cancer: a population-level comparison of techniques. Ann Surg Oncol. 2016;23:3290–3296. doi: 10.1245/s10434-016-5401-2. [DOI] [PubMed] [Google Scholar]
- 69.Edwards S.B., Leitman I.M., Wengrofsky A.J., MarleyJ Giddins, Harris E., Mills C.B. Identifying factors and techniques to decrease the positive margin rate in partial mastectomies: have we missed the mark? Breast J. 2016;22:303–309. doi: 10.1111/tbj.12573. [DOI] [PubMed] [Google Scholar]
- 70.Ahmed M., Douek M. Radioactive seed localisation (RSL) in the treatment of non-palpable breast cancers: systematic review and meta-analysis. Breast Edinb Scotl. 2013;22:383–388. doi: 10.1016/j.breast.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 71.Ahmed M., van Hemelrijck M., Douek M. Systematic review of radioguided versus wire-guided localization in the treatment of non-palpable breast cancers. Breast Canc Res Treat. 2013;140:241–252. doi: 10.1007/s10549-013-2547-5. [DOI] [PubMed] [Google Scholar]
- 72.Elmore L.C., Margenthaler J.A. A tale of two operations: re-excision as a quality measure. Gland Surg. 2019;8:593–595. doi: 10.21037/gs.2019.11.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Landercasper J., Attai D., Atisha D., Beitsch P., Bosserman L., Boughey J. Toolbox to reduce lumpectomy reoperations and improve cosmetic outcome in breast cancer patients: the American society of breast surgeons consensus conference. Ann Surg Oncol. 2015;22:3174–3183. doi: 10.1245/s10434-015-4759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morrow M., Abrahamse P., Hofer T.P., Ward K.C., Hamilton A.S., Kurian A.W. Trends in reoperation after initial lumpectomy for breast cancer: addressing overtreatment in surgical management. JAMA Oncol. 2017;3:1352. doi: 10.1001/jamaoncol.2017.0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chagpar A.B. Defining why the Re-excision rate dropped. Ann Surg Oncol. 2019;26:1176–1177. doi: 10.1245/s10434-019-07248-4. [DOI] [PubMed] [Google Scholar]
- 76.Parvez E., Cornacchi S.D., Hodgson N., Thoma A., Kong I., Foster G. A cosmesis outcome substudy in a prospective, randomized trial comparing radioguided seed localization with standard wire localization for nonpalpable, invasive, and in situ breast carcinomas. Am J Surg. 2014;208:711–718. doi: 10.1016/j.amjsurg.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 77.Sharek D., Zuley M.L., Zhang J.Y., Soran A., Ahrendt G.M., Ganott M.A. Radioactive seed localization versus wire localization for lumpectomies: a comparison of outcomes. Am J Roentgenol. 2015;204:872–877. doi: 10.2214/AJR.14.12743. [DOI] [PubMed] [Google Scholar]
- 78.Lindenberg M., van Beek A., Retèl V., van Duijnhoven F., van Harten W. Early budget impact analysis on magnetic seed localization for non-palpable breast cancer surgery. PloS One. 2020;15 doi: 10.1371/journal.pone.0232690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Konen J., Murphy S., Berkman A., Ahern T.P., Sowden M. Intraoperative ultrasound guidance with an ultrasound-visible clip: a practical and cost-effective option for breast cancer localization. J Ultrasound Med. 2020;39:911–917. doi: 10.1002/jum.15172. [DOI] [PubMed] [Google Scholar]
- 80.Rose A., Collins J.P., Neerhut P., Bishop C.V., Mann G.B. Carbon localisation of impalpable breast lesions. Breast Edinb Scotl. 2003;12:264–269. doi: 10.1016/s0960-9776(03)00105-x. [DOI] [PubMed] [Google Scholar]
- 81.Loving V.A., Edwards D.B., Roche K.T., Steele J.R., Sapareto S.A., Byrum S.C. Monte Carlo simulation to analyze the cost-benefit of radioactive seed localization versus wire localization for breast-conserving surgery in fee-for-service health care systems compared with accountable care organizations. AJR Am J Roentgenol. 2014;202:1383–1388. doi: 10.2214/AJR.13.11368. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y., Seely J., Cordeiro E., Hefler J., Thavorn K., Mahajan M. Radioactive seed localization versus wire-guided localization for nonpalpable breast cancer: a cost and operating room efficiency analysis. Ann Surg Oncol. 2017;24:3567–3573. doi: 10.1245/s10434-017-6084-z. [DOI] [PubMed] [Google Scholar]
- 83.Wright C.M., Moorin R.E., Saunders C., Marinovich M.L., Taylor D.B., Bourke A.G. Cost-effectiveness of radioguided occult lesion localization using 125I seeds versus hookwire localization before breast-conserving surgery for non-palpable breast cancer. Br J Surg. 2021 doi: 10.1093/bjs/znaa160. [DOI] [PubMed] [Google Scholar]
- 84.Law W., Look Hong N., Ravi A., Day L., Somani Y., Wright F.C. Budget impact analysis of preoperative radioactive seed localization. Ann Surg Oncol. 2021;28:1370–1378. doi: 10.1245/s10434-020-09071-8. [DOI] [PubMed] [Google Scholar]
- 85.Wilke L.G., Czechura T., Wang C., Lapin B., Liederbach E., Winchester D.P. Repeat surgery after breast conservation for the treatment of stage 0 to II breast carcinoma: a report from the national cancer data base, 2004-2010. JAMA Surg. 2014;149:1296. doi: 10.1001/jamasurg.2014.926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.