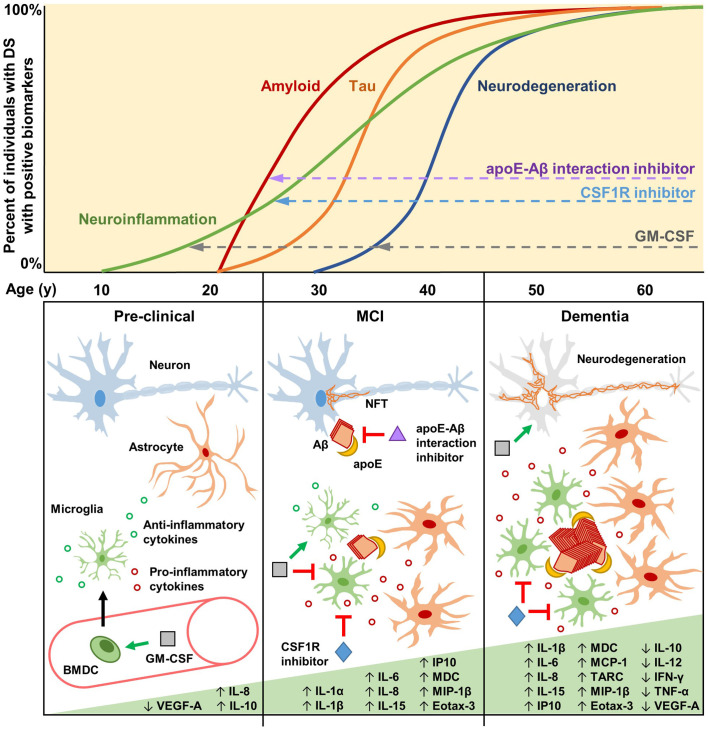
Figure 1.
Biomarkers, cytokines, and potential therapeutic mechanisms for neuroinflammation in Down syndrome (DS) and Alzheimer’s disease (AD). Biomarkers of neuroinflammation, amyloid and tau pathology, and neurodegeneration increase over the lifetimes of the vast majority of individuals with DS. Neuroinflammatory biomarkers such as GFAP may begin increasing as early as the late teenage years and progressively increase throughout life (Hendrix et al., 2021). Changes in amyloid biomarkers are detectable after 20 years of age, with APOE genotype significantly contributing to risk (Rubinsztein et al., 1999; Deb et al., 2000), likely due to apoE-catalyzed amyloid-β (Aβ) polymerization during the early seeding stages of amyloid formation (Potter and Wisniewski, 2012). Tau pathology is detectable soon after amyloid forms, but it accumulates more slowly. By the age of 40, every individual with DS has the hallmark amyloid and tau neuropathology of AD. Biomarkers of neurodegeneration, such as changes in brain volume and glucose metabolism, are identifiable after 30 years of age, proceeding and generally correlating well with tau pathology. During the pre-clinical stage, GM-CSF may have therapeutic potential by modulating the immune/neuroinflammatory cascade in order to prevent and/or delay amyloidogenesis. GM-CSF stimulates bone marrow-derived cells (BMDCs) to mobilize and extravasate to the brain, or GM-CSF may enter the brain directly, where the resulting activated microglia modulate their cytokine expression and reactivity (Bhattacharya et al., 2015a; Abe et al., 2020). During the mild cognitive impairment (MCI) stage, preceding clinical dementia, Aβ aggregates begin to form, catalyzed by apoE, which then induces intraneuronal tau hyperphosphorylation and neurofibrillary tangle (NFT) formation. Therapeutic molecules that can inhibit the apoE-Aβ interaction (e.g., Sadowski et al., 2004; Pankiewicz et al., 2014; Johnson et al., 2021a) may significantly reduce amyloid deposition, thus preventing/reducing downstream pathologies. Activated microglia and astrocytes, characterized by increased soma size and shortened processes, cluster around amyloid plaques and increase the expression of interleukins and other pro-inflammatory cytokines. At this stage, GM-CSF may act to maintain microglia in a non-activated state, promote anti-inflammatory cytokine expression, and reduce pro-inflammatory cytokine expression (Ahmed et al., 2021; Potter et al., 2021). CSF1R inhibitors may also be beneficial at this stage to reduce the numbers of activated microglia while maintaining quiescent microglia that play important roles in immune surveillance and brain homeostasis (Johnson et al., 2021b). The clinical dementia stage is characterized by neurodegeneration due to widespread NFT formation and chronic neuroinflammation that persists through the mid-40s, 50s, and 60s, during which a majority of individuals with DS have clinical dementia. Some inflammatory markers are reported to decrease during this stage, possibly due to cellular exhaustion and degeneration (Flores-Aguilar et al., 2020). GM-CSF may therapeutically modify neurodegeneration via neuroprotective effects, while CSF1R inhibitors may reduce the numbers and effects of chronically activated microglia.