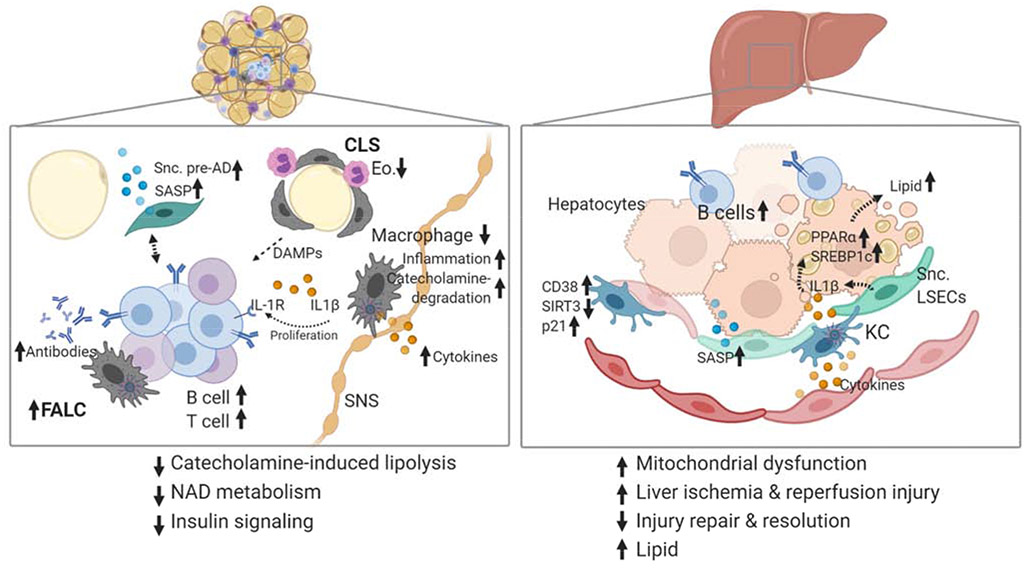
Figure 3. Aged tissue contains inflammatory and senescent cells contributing to metabolic dysfunction.
Schematic to highlight aged tissue. Liver and adipose tissue are two critical metabolic tissues that show alterations in immune cells, metabolic signaling and senescence. (Left) White visceral adipose tissue is the first to show elevated NLRP3 inflammasome activation, accumulating B and T cells in fat-associated lymphoid clusters and elevated levels of senescent cell burden. NLRP3-expressing macrophages are found in crown-like-structures (CLS), near sympathetic nerves (SNS), and fat-associated lymphoid structures (FALCs). CLS also have eosinophils (Eo.) in close contact, which support tissue resident macrophages. Dying adipocytes in CLS provide a source of damageassociated molecular patterns that activate macrophages and drive FALC accumulation. B cells in FALCs secrete antibodies, express the IL-1 receptor (IL-1R) and respond to NLRP3-induced IL1β by proliferation. Macrophages near SNS upregulate catecholamine degradation and inflammatory genes driving impairing of lipolysis. Senescent preadipocytes (Snc. Pre-AD) secrete SASP factors and interact with immune cells in ways that are not yet clear. (Right) B cells are increased in aged liver. Aged Kupffer cells (KC) upregulate the NADase, CD38, and senescence marker p21, but have lower levels of SIRT3, contributing to impaired NAD levels in liver. Mitochondrial DNA Cytokines from immune cells and SASP factors from senescent cells are increased in aged tissues. IL1β is a SASP factor secreted from KCs and Senescent liver sinusoidal endothelial cells (Snc. LSECs) which increases lipid accumulation via PPARα and SREBP1c in hepatocytes.