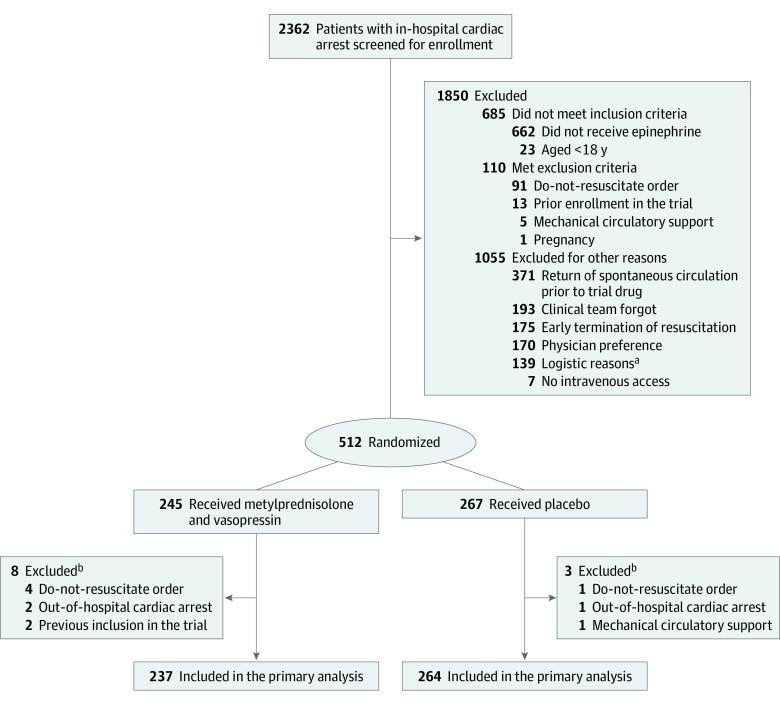
Figure 1. Screening and Randomization in the VAM-IHCA Trial of Methylprednisolone and Vasopressin for In-Hospital Cardiac Arrest.
aLogistic reasons included not enough personnel (n = 61), no study drug available (n = 45), inability to obtain surrogate consent (n = 1), and other (n = 32), which included patients isolated with COVID-19.
bPatients who were excluded after receiving the trial drugs had inclusion/exclusion criteria not known at the time of the cardiac arrest.