Figure 2.
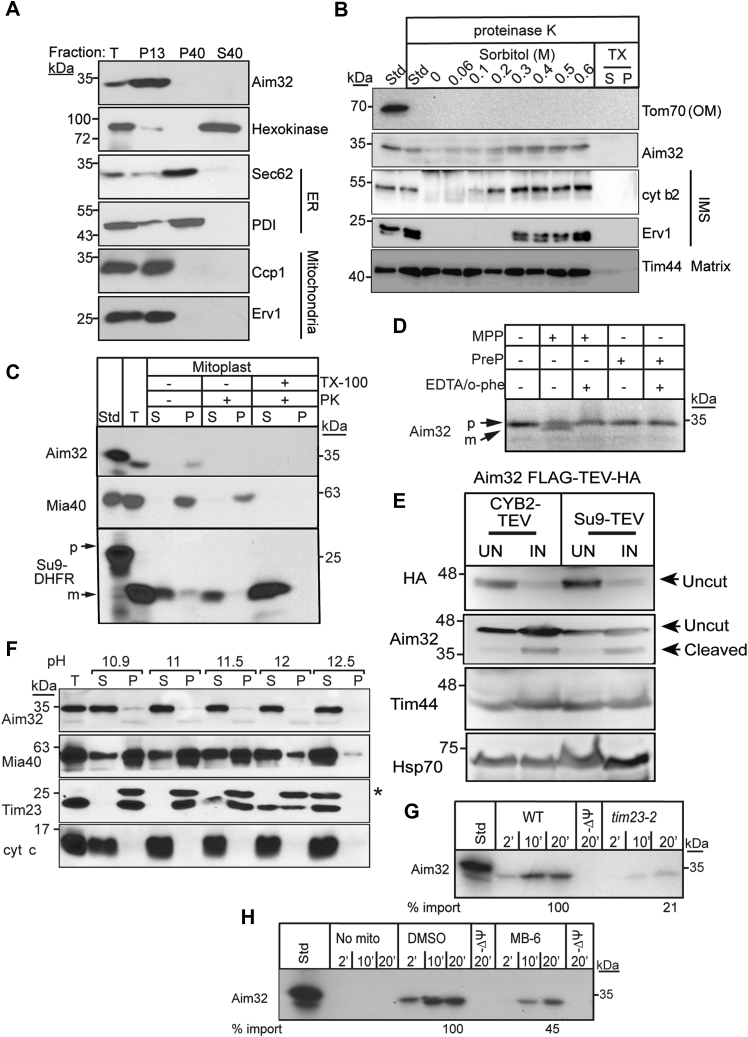
Aim32 is a soluble protein of the mitochondrial IMS and matrix, and the import is facilitated by the TIM23 translocon.A, the WT strain was grown in YPEG at 30 °C and converted to spheroplasts. The total homogenate (T) was fractionated into mitochondria (P13), microsomes (P40), and cytoplasm (S40). An equal amount of each fraction was separated by SDS-PAGE and analyzed by immunoblot using the indicated antibodies. Markers include hexokinase (cytosol), Sec62 and PDI (ER), and Ccp1 and Erv1 (mitochondria). B, mitochondria were incubated in 20 mM Hepes-KOH, pH 7.4, 100 μg/ml proteinase K, and the indicated sorbitol concentrations at 4 °C for 30 min, followed by addition of 1 mM PMSF. After centrifugation, the pellet was analyzed by SDS-PAGE and immunoblotted with antibodies against the indicated proteins. C, import of radiolabeled Aim32 into WT mitochondria was followed by osmotic shock (final concentration 20 mM Hepes-KOH, pH 7.4, 100 μg/ml proteinase K [PK] and 0.06 M sorbitol, termed Mitoplast) as described in panel B. Su9-DHFR (matrix-targeted) and Mia40 (IMS-targeted) were used as controls. D, mitochondrial processing peptidase (MPP) cleavage assay was performed with the addition of 10 μg recombinant MPP to radiolabeled Aim32, and the samples were resolved on a 12% Tris-Tricine gel. As a negative control, 10 μg recombinant presequence protease (Cym1) was added to radiolabeled Aim32. 5 mM EDTA and 2 mM o-phenanthroline were added to inhibit activity of MPP. Samples were separated by SDS-PAGE and visualized using autoradiography. E, Δaim32 cells expressing Aim32-FLAG-TEV-HA were transformed with plasmids expressing matrix-localized Su9-TEV protease or IMS-localized CYB2 [1-220]-TEV protease. Cells were grown in minimal media supplemented with 2% galactose (IN) or 2% sucrose (UN) and harvested in the midlog phase. Whole-cell extracts were analyzed by immunoblotting against proteins Aim32, Tim44, Hsp70, and HA. Arrows indicate uncut and cleaved Aim32 FLAG-TEV-HA proteins. F, WT mitochondria were analyzed by alkali extraction using 0.1 M carbonate at the indicated pH. Equal amounts of the pellet (P) and TCA-precipitated supernatant (S) fractions from 50 μg mitochondria were resolved by SDS-PAGE and immunoblotted for the indicated mitochondrial markers. The asterisk indicates nonspecific band. G, radiolabeled Aim32 was imported into WT and tim23-2 mutant mitochondria in the indicated time course. Nonimported precursor was removed by protease treatment, and the imported Aim32 was analyzed by SDS-PAGE and autoradiography. “-Ψ” indicates import when the membrane potential was dissipated with 1 μM valinomycin. A 10% standard (Std) from the translation reaction was included. Import reactions were quantitated using ImageJ software; 100% was set as the amount of precursor that imported into WT mitochondria at the endpoint of the time course. A representative gel is shown (n = 3). H, as in panel G, Aim32 was imported into WT mitochondria in the presence and absence of a ΔΨ and 25 μM of MitoBLoCK-6 (42) or a vehicle control [1% dimethyl sulfoxide (DMSO)]. Aim32, altered inheritance of mitochondria 32; ER, endoplasmic reticulum; IMS, intermembrane space; IN, induced; m, mature; MIA, mitochondrial intermembrane space assembly; OM, outer membrane; p, precursor; UN, uninduced.