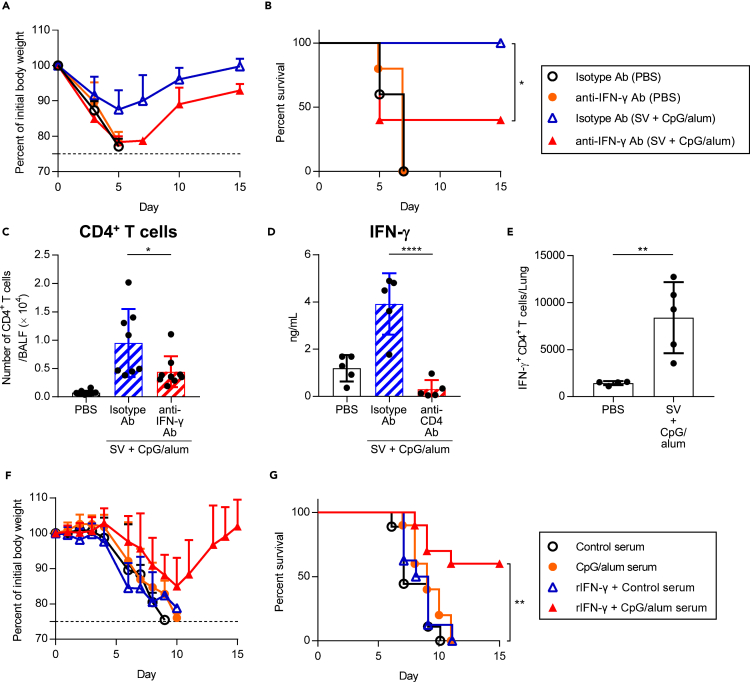
Figure 4.
Requirement of IFN-γ for cross-protection
(A–C) After treatment with PBS or immunization with SV plus CpG/alum, anti-IFN-γ antibody or isotype antibody was injected into mice before 1.2 × 103 TCID50 PR8 challenge. We monitored (A) percentages of initial body weights and (B) survival for the next 15 days. (C) Five days after PR8 challenge, the number of CD4+ T cells in BALF was measured.
(D) After treatment with PBS or immunization with SV plus CpG/alum, anti-CD4 antibody or isotype antibody was injected into mice before and during the 1.2 × 103 TCID50 PR8 challenge. Five days after the PR8 challenge, the level of IFN-γ in BALF was measured.
(E) After treatment with PBS or immunization with SV plus CpG/alum, mice were challenged with 1.2 × 103 TCID50 PR8. Four days after challenge, mice were treated with Brefeldin A intraperitoneally to block cytokine secretion, followed by harvesting of the lung after 6 h. Single cell suspensions were prepared and intracellular IFN-γ was analyzed by flow cytometry.
(F and G) Mixture of 1.2 × 10 TCID50 PR8 and 2 fold diluted serum from PBS-treated control mice or SV plus CpG/alum-immunized mice was transferred into naive mice intranasally. These mice were treated with recombinant IFN-γ, and we monitored (F) percentages of initial body weights and (G) survival for the next 15 days. (A, B, D and E) n = 5, (C, F and G) n = 8–10 per group. (A, C, D, E and F) Data are means ± SD. (B) ∗p<0.05 as indicated by comparing Kaplan–Meier curves using the logrank test. (C and D) ∗p<0.05, ∗∗∗∗p<0.0001 as indicated by Tukey’s test. (E) ∗∗p<0.01 as indicated by Student’s ttest. (G) ∗∗p<0.01 between recombinant IFN-γ plus serum from SV plus CpG/alum group vs. serum from SV plus CpG/alum group as indicated by comparing Kaplan–Meier curves using the logrank test. See also Figures S5 and S6.