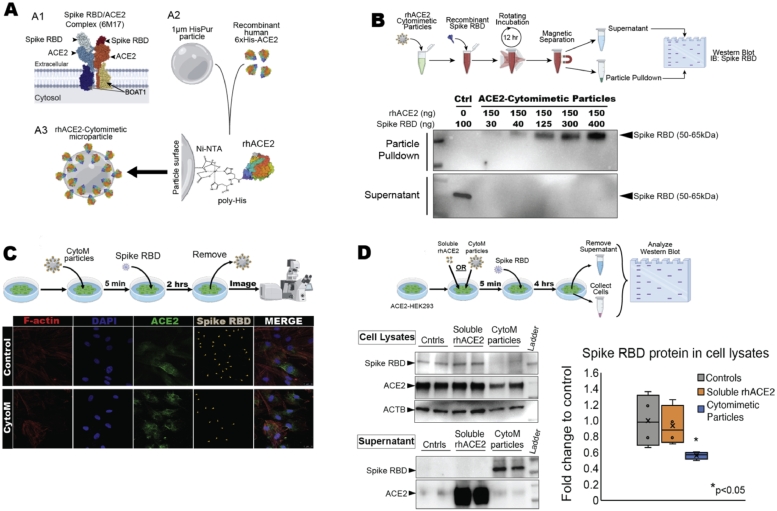
Fig. 1.
rhACE2-Cytomimetic particles and in vitro studies, (A) rhACE2-Cytomimetic particles fabrication showing the known SARS-CoV-2 interactions with ACE2 (A1, PDB: 6M17, [6]), components and functionalization chemistry utilized in this study (A2), and final conceptualized rhACE2-Cytomimetic particles (A3). (B) rhACE2-Cytomimetic particles binding capacity experiment (top) and western blot showing loading capacity of SARS-CoV-2 spike protein receptor biding domain (RBD) onto rhACE2-Cytomimetic particles (bottom). (C) rhACE2-Cytomimetic particles in vitro testing on primary human epithelial cell cultures (top), and immunohistochemistry images for F-actin, DAPI, ACE2, and Spike RBD (gray with orange arrowheads) present on human epithelial cells pre-incubated with HisPur beads (control) or rhACE2-Cytomimetic particles and exposed to SARS-CoV-2 Spike RBD protein for 2 h (bottom). (D) Comparative study between rhACE2-Cytomimetic particles and soluble rhACE2 in vitro testing on primary ACE2 overexpressing HEK293 cell cultures, human epithelial cell cultures (top). Western blot images of RBD, ACE2, and ACTB in cell lysate and supernatant fractions, and quantification of spike RBD protein in cell lysate fraction (bottom, *p < 0.05).