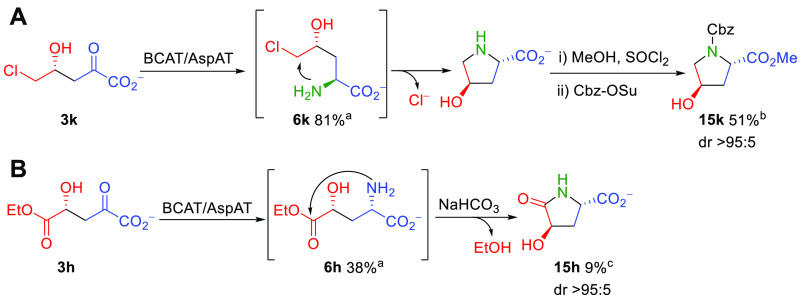
Scheme 8. (A) Formation of (2S,4R)-(−)-trans-4-Hydroxyproline Derivative 15k by Tandem Enzymatic Aldol Reaction/Transamination and Intramolecular Nucleophilic Substitution with the Amine Group and (B) Formation of γ-Hydroxypyroglutamic Acid (15h) by Intramolecular Aminolysis of the Ethyl Ester Group under Basic Conditions.
Percentage of product formed determined by HPLC.
Isolated yield from 1k.
Isolated yield from 1h. The material contained l-Asp and l-Glu as major impurities.
The stereochemistry of 15k was established unequivocally by a comparison with authentic samples (see Figure S26).