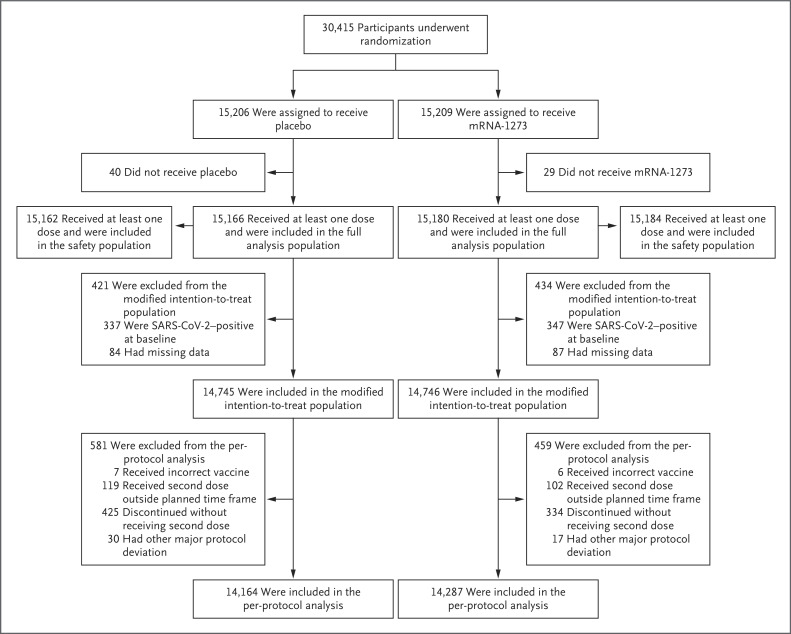
Figure 1. Randomization and Analysis Populations.
Eight participants, including six with major protocol deviations and two who erroneously underwent randomization twice, were excluded from the original randomization population (30,423 participants) and from all analysis sets. The full analysis population comprised all participants who had undergone randomization and received at least one injection; the modified intention-to-treat population included participants in the full analysis population who had no immunologic or virologic evidence of previous Covid-19 (i.e., had both a negative nasopharyngeal swab specimen and a negative anti-nucleocapsid antibody test result) at day 1 before the first injection; and the per-protocol population consisted of all participants in the modified intent-to-treat population who received planned injections according to the schedule and had no major protocol deviations that affected key trial data. The safety population included all participants who had undergone randomization and received at least one injection; this population was used for all safety analyses except the analysis for solicited adverse events. For safety analyses, participants were evaluated according to the injection received. Three participants assigned to the mRNA-1273 group received two doses of placebo and were included in the placebo safety population, and seven participants assigned to the placebo group received one or two doses of mRNA-1273 and were included in the mRNA-1273 safety population. The data cutoff date was March 26, 2021.