Figure 10.
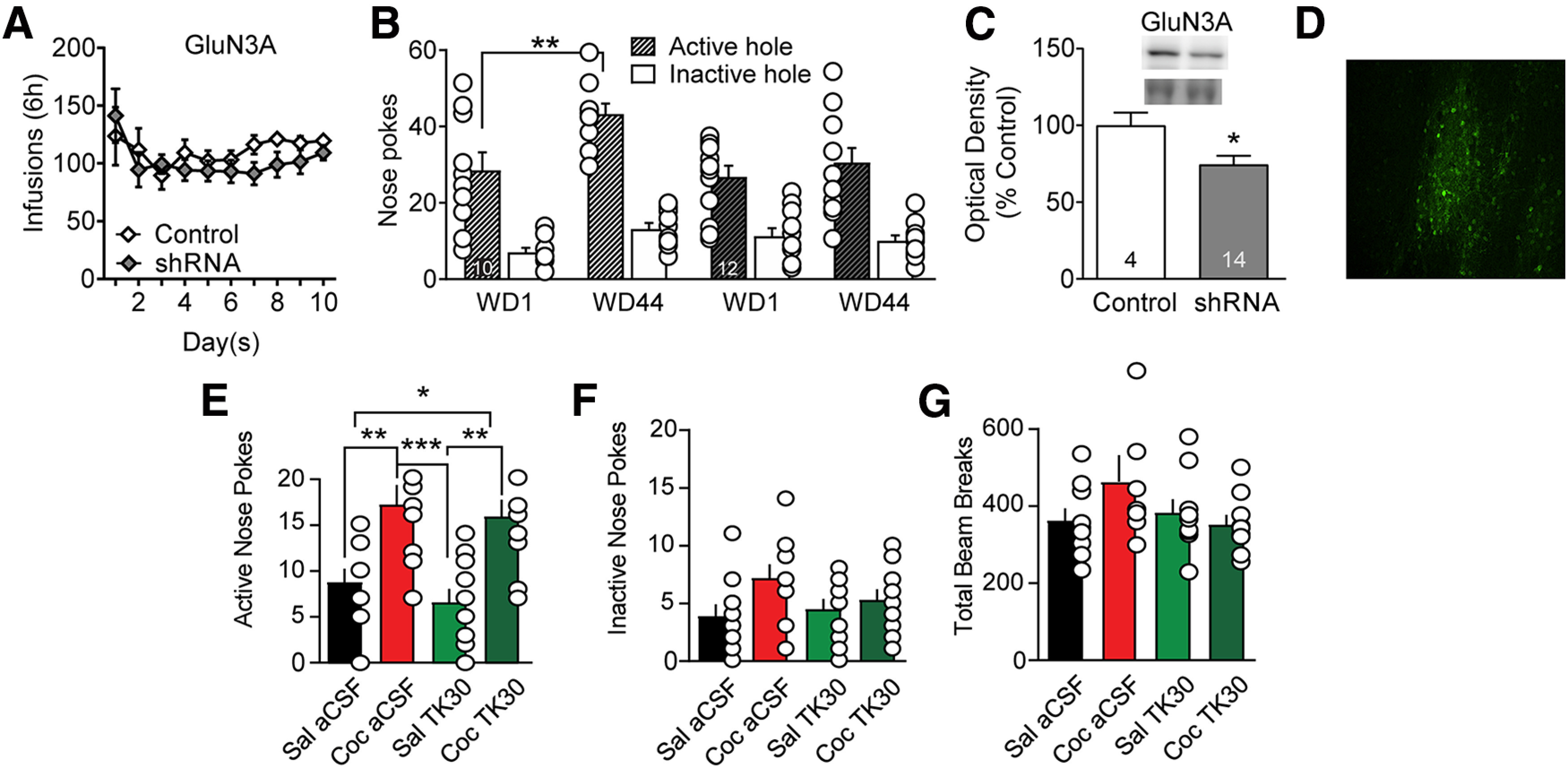
Viral knockdown of GluN3A in NAc core prevents incubation of cocaine craving but acute intra-NAc infusion of the GluN3 antagonist TK30 in late withdrawal does not prevent expression of incubated craving. A, GluN3A knockdown before cocaine self-administration did not impact drug taking during the training period. Mixed-effect analysis: self-administration day, F(9,180) = 2.329, p = 0.0167; active versus control virus, F(1,20) = 0.8108, NS; interaction (self-administration day vs active/control virus), F(9,180) = 0.8880, NS. No significant post hoc tests (Holm–Sidak). B, GluN3A knockdown prevented incubation of craving, measured as increased cue-induced responding in the previously active nose-poke hole on WD44 versus WD1. With a three-way repeated-measures ANOVA (nose-poke hole × virus condition × WD), we observed a significant WD × virus condition interaction (F(1,40) = 4.144, p = 0.048). In rats receiving control virus, Bonferroni-corrected planned comparisons demonstrated a significant increase in nose-pokes from WD1 to WD44 (p = 0.006). Animals in the active shRNA virus group did not show incubation (p = 0.323). The same conclusion, namely, that the active shRNA virus blocked incubation, was supported if we conducted separate two-way repeated-measures ANOVAs for active and inactive hole responses (active hole: significant main effects for WD [F(1,39) = 5.564, p = 0.0234] and virus condition [F(1,39) = 5.751, p = 0.0214]; inactive hole: no significant main effects). C, Viral efficacy indicated by decreased GluN3A protein expression in NAc core of drug-naive rats. Upper bands represent GluN3A, and lower bands represent a prominent Ponceau S-stained band from the same lane; Ponceau staining in the entire lane was our measure of protein loading. Unpaired t test: control versus GluN3 shRNA (t(16) = 2.169, p < 0.05). D, Visualization of virus expression based on GFP fluorescence in NAc core of a representative drug-naive rat. Scale bar, 50 µm. E–G, Microinjection of TK30 directly into the NAc core after protracted withdrawal from extended-access cocaine self-administration does not prevent expression of incubated cocaine seeking. Rats self-administered saline or cocaine as in previous experiments. Each rat underwent two cue-induced seeking tests (WD35 and WD42). One hour before the first seeking test, aCSF (control) or TK30 (GluN3-selective antagonist; 30 μm in 0.5 µl/hemisphere) was injected bilaterally into the NAc core of 10 rats (5 received aCSF and 5 received TK30). Seven days later (WD42), using a crossover design, aCSF or TK30 was injected bilaterally into the NAc core of the same rats 1 h before the second seeking test. This yielded four experimental groups: Sal aCSF, Coc aCSF, Sal TK30, and Coc TK30 (n = 10 observations per group). Compared with aCSF injection, TK30 injection had no effect on active hole responding (E), inactive hole responding (F), or locomotor activity during the test (G) in either saline or cocaine rats; that is, cocaine rats expressed incubation regardless of whether they were pretreated with aCSF or TK30. Thus, activation of GluN3-containing NMDARs during a seeking test is not required for the expression of incubation, although their synaptic incorporation during cocaine withdrawal is necessary for incubation to occur (B). We note that active hole responding in the cocaine late withdrawal groups in E is lower than for cocaine late withdrawal groups in B. This is likely because the former groups received an intracranial injection immediately before the test, which typically lowers active hole responding. E–G, Data were analyzed using one-way ANOVA with Holm–Sidak post hoc tests. E, Active hole: F(3,36) = 8.744, p = 0.0002; saline aCSF versus cocaine aCSF, p = 0.0093; saline aCSF versus cocaine TK30, p = 0.0342; cocaine aCSF versus saline TK30, p = 0.0008; saline TK30 versus cocaine TK30, p = 0.0035. F, Inactive hole: F(3,36) = 2.034, p = 0.1265. G, Locomotor activity: F(3,36) = 2.034; p = 0.1265. *p < 0.05. **p < 0.01. ***p < 0.001.
