Abstract
Objective
Sleep and pain-related experiences are consistently associated, but the pathways linking these experiences are not well understood. We evaluated whether pain catastrophizing and arthritis self-efficacy mediate the association between sleep disturbance and osteoarthritis (OA) symptom severity in patients with knee OA.
Methods
We analyzed cross-sectional baseline data collected from Veterans Affairs (VA) patients enrolled in a clinical trial examining the effectiveness of a positive psychology intervention in managing pain from knee OA. Participants indicated how often in the past two weeks they were bothered by trouble falling asleep, staying asleep, or sleeping too much. We used validated scales to assess the primary outcome (OA symptom severity) and potential mediators (arthritis self-efficacy and pain catastrophizing). To test the proposed mediation model, we used parallel multiple mediation analyses with bootstrapping, controlling for sociodemographic and clinical characteristics with bivariate associations with OA symptom severity.
Results
The sample included 517 patients (Mage = 64 years, 72.9% male, 52.2% African American). On average, participants reported experiencing sleep disturbance at least several days in the past two weeks (M = 1.41, SD = 1.18) and reported moderate OA symptom severity (M = 48.22, SD = 16.36). More frequent sleep disturbance was associated with higher OA symptom severity directly (b = 3.08, P <0.001) and indirectly, through higher pain catastrophizing (b = 0.60, 95% confidence interval [CI] = 0.20 to 1.11) and lower arthritis self-efficacy (b = 0.84, 95% CI = 0.42 to 1.42).
Conclusions
Pain catastrophizing and arthritis self-efficacy partially mediated the association between sleep disturbance and OA symptom severity. Behavioral interventions that address pain catastrophizing and/or self-efficacy may buffer the association between sleep disturbance and OA symptom severity.
Keywords: Sleep, Self-Efficacy, Pain Catastrophizing, Pain, Veterans, Osteoarthritis
Introduction
Pain is a complex phenomenon influenced by a host of biological, psychological, and social factors [1]. Previous reports have established that there is a bidirectional relationship between sleep and pain [2–6]. Although it is well established that sleep and pain are associated, the factors that contribute to the sleep–pain relationship are not fully understood [2]. Based on their work investigating Cognitive Behavioral Therapy for insomnia (CBT-I) in older patients with osteoarthritis (OA), Vitiello and colleagues proposed that sleep and pain are linked via a positive feedback loop [7]. Specifically, they posit that dysregulated sleep schedules lead to nonrestorative sleep, reduced pain thresholds, and more negative pain-related emotions and cognitions, which, in turn, lead to increased pain and activity limitations and further exacerbation of sleep difficulties [7]. Pain catastrophizing, the tendency to approach pain with negative cognitions, and pain-related self-efficacy, expectations about one’s ability to cope with pain, are two pain-related cognitions that have been uniquely linked to both sleep [8,9] and pain outcomes [10,11]. Though each represents pain-related cognitions, greater pain catastrophizing is consistently associated with poorer outcomes (e.g., heightened pain severity, greater functional impairment) [11], whereas greater pain-related self-efficacy is consistently associated with better outcomes (e.g., lower pain severity, lower levels of functional impairment, enhanced quality of life) [10,12,13]. Consistent with the conceptual model proposed by Vitiello and colleagues [7], pain catastrophizing and pain-related self-efficacy may serve as mediating factors linking sleep and pain-related experiences such as pain severity and activity limitations; however, their mediating role has not been empirically demonstrated.
Observational research in individuals with chronic pain has indicated that poorer habitual sleep is associated with higher levels of catastrophizing and that, at the daily level, worse previous-night sleep is associated with worse next-morning pain catastrophizing [9,14–16]. Experimental studies have corroborated observational findings linking poor sleep and pain catastrophizing [17–20]. Despite also representing pain-related cognitions and being a unique predictor of pain-related outcomes [13], pain-related self-efficacy has received less attention as a correlate of sleep. Sturgeon and Zautra proposed a paradigm for understanding pain coping that emphasizes consideration of factors that contribute not only to vulnerability but also to resilience [21]; the same consideration is arguably necessary when investigating influences on pain experiences. Based on existing literature linking better sleep with better cognitive and affective functioning [22,23], poor sleep may deplete adaptive resources such as one’s sense of self-efficacy. Accordingly, emerging observational [24,25] and experimental [19] research has supported the association of sleep with pain-related self-efficacy.
In sum, it has been established that sleep and pain experiences are bidirectionally related in individuals with chronic pain, with current evidence suggesting that sleep may be a more reliable predictor of pain than pain is of sleep [2,6]. Pain catastrophizing and pain-related self-efficacy are important predictors of pain experiences [10,11] and have been associated with sleep [8,9], but have not been examined as pathways linking sleep and pain-related experiences. The concurrent consideration of both pain catastrophizing and pain-related self-efficacy as mediating pathways is essential to further our understanding of both deteriorative and restorative pain-related processes. The objective of the current analysis was to determine if pain catastrophizing and arthritis self-efficacy mediate the association of sleep disturbance and pain-related experiences in a sample of African American and white middle-aged to older US military veterans with knee OA. We hypothesized that more frequent sleep disturbance would be associated with higher levels of OA symptom severity both directly and indirectly, through higher levels of pain catastrophizing and lower levels of arthritis self-efficacy.
Method
Participants and Procedure
The current study is a secondary analysis of data collected during the baseline visit of the Staying Positive with Arthritis (SPA) study (ClinicalTrials.gov Identifier: NCT02223858), a randomized controlled trial examining the impact of a positive psychological intervention on racial disparities in pain in US military veterans [26,27]. To participate in the SPA study, individuals were required to be age 50 years or older; identify their race as non-Hispanic African American or non-Hispanic white; report symptomatic knee pain consistent with knee OA (defined by the Osteoarthritis Initiative [28]); endorse pain severity ratings ≥4 on a 0–10 scale; receive primary care at one of two participating Veterans Affairs (VA) medical centers; and have the ability to speak, read, and write in English. Exclusion criteria included significant problems with hearing, vision, or memory; diagnoses of arthritis other than OA or degenerative arthritis; treatment for cancer in the past three years; receipt of steroid injection into one or both knees in the past three months; replacement of one or both knees in the past three months; plans to undergo knee replacement in the next six months; inability to complete study procedures; lack of a reliable telephone number; or two or more items answered incorrectly on a six-item cognitive screening instrument [29].
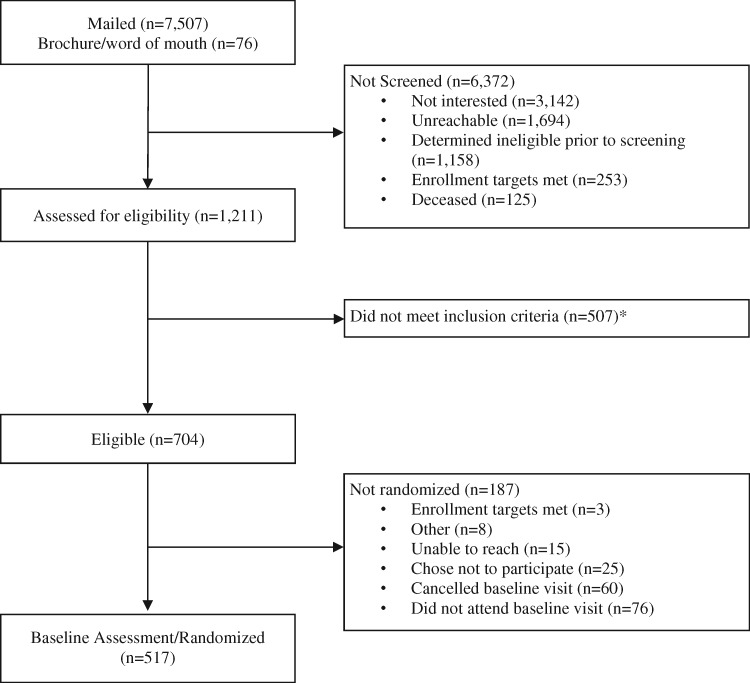
Prospective participants were recruited from the VA Pittsburgh Healthcare System and the Corporal Michael J. Crescenz VA Medical Center in Philadelphia. Recruitment procedures, which are described in detail elsewhere [26,27], involved distributing study brochures at participating medical centers and via targeted mailings based on basic eligibility criteria ascertained from VA electronic health records (EHR). Prospective participants completed an in-depth telephone screening to determine study eligibility. Eligible participants provided written informed consent and completed a battery of measures administered by research personnel during a baseline visit held at their VA medical center. The current analysis included baseline measures collected from 517 SPA study participants (Figure 1; Supplementary Data). The study procedures were approved by the VA Central Institutional Review Board.
Figure 1.
Study flow diagram. *Reasons prospective participants did not meet exclusion criteria are listed in the Supplementary Data.
Study Measures
Primary Predictor: Sleep Disturbance
We assessed sleep disturbance with a single item from the Patient Health Questionnaire-8 (PHQ-8) [30], which asked participants to indicate how often in the preceding two weeks they were bothered by “trouble falling or staying asleep, or sleeping too much” (0 = not at all, 1 = several days, 2 = more than half the days, and 3 = nearly every day). This item has previously demonstrated concurrent validity with the Insomnia Severity Index [31,32]. We treated sleep disturbance as a continuous variable in statistical analysis.
Primary Outcome: OA Symptom Severity
We measured OA symptom severity using the Western Ontario and McMaster (WOMAC) Osteoarthritis Index [33]. The WOMAC Index includes subscales for the dimensions of pain (five items), physical function (17 items), and stiffness (two items), which can be combined into a composite score indicative of overall OA symptom severity [33]. For the pain and physical function items, respondents are asked to indicate the extent to which they experience pain or difficulty while completing daily tasks. For the stiffness items, respondents are asked to indicate the extent of stiffness they experience after first awakening in the morning and later in the day. All responses are based on experiences in the past seven days and use a five-point Likert-type scale (0 = none, 1 = mild, 2 = moderate, 3 = severe, 4 = extreme). We calculated an overall score of OA symptom severity (WOMAC total) by summing the scores of the three subscales and converting the summed score to a 0–100 scale. Total scores were calculated if there were no missing subscale scores; pain subscale scores (Cronbach’s α = 0.80 in the current sample) were calculated if there was no more than 1 missing pain item, physical function subscale scores (Cronbach’s α = 0.94 in the current sample) were calculated if there were no more than three missing physical function items, and stiffness subscale scores (Cronbach’s α = 0.68 in the current sample) were calculated if there was no more than one missing stiffness item.
Proposed Mediators
We measured pain catastrophizing using the Pain Catastrophizing Scale (PCS) [34]. This 13-item scale prompts respondents to indicate the degree to which they have thoughts and feelings characterized by rumination, magnification, and helplessness in the context of pain (0 = not at all, 1 = to a slight degree, 2 = to a moderate degree, 3 = to a great degree, 4 = all the time). PCS scores were derived by summing item responses, yielding a total score ranging from 0 to 52 (Cronbach’s α = 0.94 in the current sample). Scores were calculated if there were no more than three missing items. Higher scores indicate higher levels of catastrophic thinking.
We measured self-efficacy using the Arthritis Self-Efficacy Scale [35]. The Arthritis Self-Efficacy Scale is a 20-item measure in which respondents indicate on a 10-point Likert scale their degree of certainty in their ability to cope with symptoms or consequences of arthritis (1 = very uncertain, 10 = very certain). Responses are used to derive subscale scores related to pain, function, and other symptoms. Subscale scores were created by averaging item responses, yielding scores ranging from 1 to 10, where higher scores are indicative of greater perceived efficacy. Pain (Cronbach’s α = 0.82 in the current sample) and other symptoms (Cronbach’s α = 0.88 in the current sample) subscale scores were calculated if there was no more than one item missing; function subscale scores (Cronbach’s α = 0.87 in the current sample) were calculated if there were no more than two items missing. Consistent with prior studies [36,37], we then calculated a total arthritis self-efficacy score by summing the pain, function, and other symptoms subscale scores, yielding scores ranging from 3 to 30 (Cronbach’s α = 0.79 in the current sample).
Sociodemographic and Clinical Characteristics
Participant characteristics were measured using self-report questionnaires and EHR data. Self-reported characteristics included gender, age, race, income, level of education, and current use of pharmacological treatments for OA [28]. Self-report items from Behavioral Risk Factor Surveillance System Questionnaires [38] were used to assess history of depression and anxiety. Body mass index (BMI), radiographic evidence of OA, and history of insomnia or sleep-related breathing disorder diagnoses were collected from the EHR (see the Supplementary Data for sleep disorder diagnostic codes).
Statistical Analysis
Statistical significance was set at the 0.05 probability level, with analyses reported from SPSS, version 25. We examined bivariate correlations among sleep disturbance, pain catastrophizing, arthritis self-efficacy, and OA symptom severity and evaluated the strength of bivariate correlations using Cohen’s criteria [39]. The PROCESS macro for SPSS 2.16.3 [40] was used to test several parallel multiple mediation models. The PROCESS macro assesses parallel multiple mediation models using ordinary least squares path analysis and bootstrapping techniques [40]. We estimated 95% bias-corrected bootstrap confidence intervals for total indirect effects using 10,000 bootstrap samples. A significant indirect effect is indicated when the 95% confidence interval (CI) associated with the bootstrapped estimates does not contain 0. For our main analysis, we assessed a parallel multiple mediation model in which sleep disturbance was the primary predictor, pain catastrophizing and arthritis self-efficacy (Arthritis Self-Efficacy Scale total score) were mediators, and OA symptom severity (WOMAC total score) was the primary outcome. Given the cross-sectional nature of our data, we also tested an alternative model to determine whether the mediational pattern of findings held with OA symptom severity as the primary predictor and sleep disturbance as the primary outcome.
Based on established associations with pain, we considered the following variables for inclusion as covariates in the parallel multiple mediation models: age, gender, BMI, race, education, income, history of anxiety, history of depression, radiographic evidence of knee OA, self-reported use of opioid analgesics for knee pain, self-reported receipt of steroid injection for knee pain in past six months, history of insomnia disorder diagnoses, and history of breathing-related sleep disorder diagnoses. We included variables as covariates in the parallel multiple mediation models if they had significant (P < 0.05) bivariate associations with WOMAC total scores.
We conducted sensitivity analyses to determine whether the relationships observed in the main analysis were consistent when distinct pain-related experiences were considered separately. Specifically, we first repeated the main analysis replacing the total self-efficacy and WOMAC scores with the Arthritis Self-Efficacy Scale pain subscale score and WOMAC pain subscale score, respectively. Second, we replaced the total self-efficacy and WOMAC scores with the Arthritis Self-Efficacy Scale function subscale score and WOMAC physical function subscale score, respectively.
Results
Sample Characteristics
Among the 517 participants in this predominantly male (72.9%) sample, the average age was 63.7 years. Roughly half of the sample identified as African American and half as white. Over one-third had a history of depression (44.1%) or anxiety (38.9%), and nearly one-third were using an opioid analgesic for their knee pain at the time of the study (31.7%) (see Table 1 for additional sample characteristics). At the bivariate level, OA symptom severity was significantly associated with age, BMI, race, education, income, history of depression, history of anxiety, radiographic evidence of OA, and current use of opioid analgesics (all P < 0.05) (Supplementary Data). Thus, these variables were included as covariates in multiple mediation analyses.
Table 1.
Sample characteristics (N = 517)
| Characteristic | M | SD |
|---|---|---|
| Age | 63.72 | 8.47 |
| Body mass index | 32.18 | 6.45 |
|
| ||
| Characteristic | n | % |
|
| ||
| Male gender | 377 | 72.9 |
| African American race | 270 | 52.2 |
| Education | ||
| High school or less | 144 | 27.9 |
| At least some college | 373 | 72.1 |
| Annual income | ||
| <$20,000 | 127 | 26.0 |
| ≥$20,000 | 362 | 74.0 |
| History of depression | 228 | 44.1 |
| History of anxiety | 201 | 38.9 |
| Radiographic evidence of osteoarthritis* | 330 | 63.8 |
| Currently utilizing opioid analgesics | 164 | 31.7 |
| Steroid injection for knee pain in past 6 mo | 47 | 9.1 |
| History of insomnia disorder* | 44 | 8.5 |
| History of sleep-related breathing disorder* | 107 | 20.7 |
Derived from Veterans Affairs Electronic Health Records.
Relations Among Sleep Disturbance, OA Symptom Severity, and Potential Mediators
On average, participants reported having sleep disturbance between “several days” and “more than half the days” in the last two weeks (M = 1.41, SD = 1.18; full response distribution: 30.2% not at all, 25.9% several days, 16.8% more than half the days, and 27.1% nearly every day). Participants also reported moderate OA symptom severity (M = 48.22, SD = 16.36) (Table 2). As shown in the bivariate correlation matrix in Table 2, sleep disturbance showed statistically significant small to moderate positive associations with pain catastrophizing and OA symptom severity and a significant small to moderate negative association with arthritis self-efficacy. Pain catastrophizing and arthritis self-efficacy showed statistically significant moderate positive and negative associations, respectively, with OA symptom severity.
Table 2.
Descriptive statistics and Pearson correlations among sleep, pain beliefs, and OA symptom severity variables
| Variable | Range | M | SD | 1. | 2. | 3. | 4. |
|---|---|---|---|---|---|---|---|
| 1. Sleep disturbance | 0–3 | 1.41 | 1.18 | — | |||
| 2. Pain catastrophizing | 0–52 | 19.11 | 12.35 | 0.38** | — | ||
| 3. Arthritis self-efficacy | 4.2–30 | 19.31 | 5.14 | –0.30** | –0.60** | — | |
| 4. OA symptom severity | 2.08–95.83 | 48.22 | 16.36 | 0.30** | 0.46** | –0.53** | — |
OA=osteoarthritis.
P < 0.001, two-tailed.
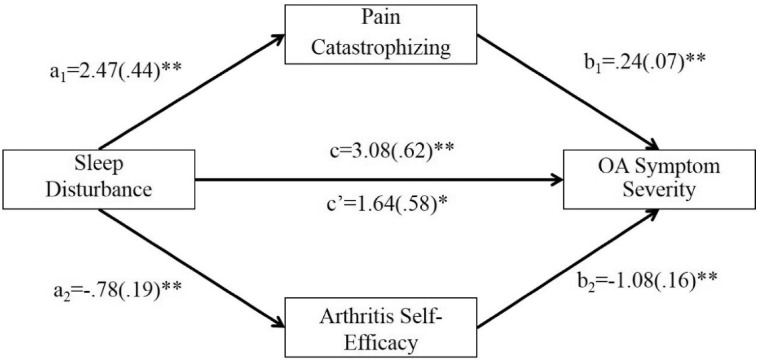
Results from the parallel multiple mediation model (Figure 2 and Table 3) indicated that frequency of sleep disturbance was significantly associated with higher levels of pain catastrophizing (a1 = 2.47, P < 0.001) and lower levels of arthritis self-efficacy (a2 = –0.78, P < 0.001). Higher levels of catastrophizing (b1 = 0.24, P < 0.001) and lower levels of arthritis self-efficacy (b2 = –1.08, P < 0.001) were, in turn, significantly associated with higher levels of OA symptom severity. A bias-corrected bootstrap confidence interval for the total indirect effect was entirely above 0 (95% CI = 0.83 to 2.15), indicating that pain catastrophizing and arthritis self-efficacy collectively mediated the association of sleep disturbance and OA symptom severity. Bias-corrected bootstrap confidence intervals for the specific indirect effects were similarly above zero for pain catastrophizing (a1b1 = 0.60, 95% CI = 0.20 to 1.11) and arthritis self-efficacy (a2b2 = 0.84, 95% CI = 0.42 to 1.42), evidencing specific indirect effects of sleep disturbance on OA symptom severity through pain catastrophizing and arthritis self-efficacy. Accounting for pain catastrophizing and arthritis self-efficacy, sleep disturbance remained significantly positively associated with OA symptom severity (c’ = 1.64, P = 0.01), suggesting that pain catastrophizing and arthritis self-efficacy are partial mediators of the association of sleep disturbance and OA symptom severity.
Figure 2.
Model assessing pain catastrophizing and arthritis self-efficacy as parallel mediators of the association of sleep disturbance and osteoarthritis (OA) symptom severity. Covariates in this model included age, body mass index, race, education, income, history of depression, history of anxiety, radiographic evidence of OA, and current use of opioid analgesics. Effects are expressed in an unstandardized metric. *P < 0.05; **P < 0.001.
Table 3.
Regression coefficients, standard errors, and model summary statistics for the sleep disturbance, pain catastrophizing, arthritis self-efficacy, and OA symptom severity parallel multiple mediation model (N = 478)
| Pain Catastrophizing |
Arthritis Self-Efficacy |
OA Symptom Severity |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor | Coeff. | SE | P | Coeff. | SE | P | Coeff. | SE | P | |||
| Sleep disturbance | a 1 | 2.47 | 0.44 | <0.001 | a 2 | −0.78 | 0.19 | <0.001 | c’ | 1.64 | 0.58 | 0.01 |
| Pain catastrophizing | — | — | — | — | — | — | b 1 | 0.24 | 0.07 | <0.001 | ||
| Arthritis self-efficacy | — | — | — | — | — | — | b 2 | −1.08 | 0.16 | <0001 | ||
| Constant | i M1 | 17.86 | 5.38 | 0.001 | i M2 | 28.23 | 2.30 | <0.001 | iY | 55.87 | 8.46 | <0.001 |
| R2 = 0.28 | R2 = 0.26 | R2 = 0.36 | ||||||||||
| F(10,467) = 18.06, P <0.001 | F(10,467) = 16.20, P <0.001 | F(12,465) = 21.66, P <0.001 | ||||||||||
Parallel multiple mediation analysis was conducted using ordinary least squares path analysis. Analysis adjusted for age, body mass index, race, education, income, history of depression, history of anxiety, radiographic evidence of osteoarthritis, and current use of opioid analgesics. Effects are expressed in an unstandardized metric.
OA = osteoarthritis.
Due to the cross-sectional, observational study design, we also tested an alternative model wherein OA symptom severity was the primary predictor and sleep disturbance was the outcome [40]. In the alternative model, a bias-corrected bootstrap confidence interval for the total indirect effect was entirely above 0 (95% CI = 0.002 to 0.01), indicating that pain catastrophizing and arthritis self-efficacy collectively mediated the association of OA symptom severity and sleep disturbance. A bias-corrected bootstrap confidence interval for the specific indirect effect through pain catastrophizing was above 0 (95% CI = 0.002 to 0.009), but a bias-corrected bootstrap confidence interval for the specific indirect effect through arthritis self-efficacy included 0 (95% CI = –0.002 to 0.01) (Supplementary Data). This pattern of findings suggests that OA symptom severity is linked to sleep disturbance directly and indirectly, through pain catastrophizing but not arthritis self-efficacy.
Sensitivity Analyses Examining Relations Among Sleep Disturbance, Specific OA Symptoms, and Potential Mediators
We found a consistent pattern of results in models where the total self-efficacy and WOMAC scores were replaced with symptom-specific subscales. First, when total arthritis self-efficacy and WOMAC scores were replaced with pain-specific subscale scores, bias-corrected bootstrap confidence intervals for the total indirect effect (95% CI = 0.71 to 1.89) and the specific indirect effects (pain catastrophizing: 95% CI = 0.40 to 1.43; pain self-efficacy: 95% CI = 0.12 to 0.80) were entirely above 0. Therefore, both pain catastrophizing and pain self-efficacy mediated the association of sleep disturbance and pain severity (Supplementary Data). Similarly, when total arthritis self-efficacy and WOMAC scores were replaced with function-specific subscale scores, bias-corrected bootstrap confidence intervals for the total indirect effect (95% CI = 0.62 to 2.04) and the specific indirect effects (pain catastrophizing: 95% CI = 0.36 to 1.40; self-efficacy related to function: 95% CI = 0.10 to 0.94) were entirely above 0. Therefore, both pain catastrophizing and functional self-efficacy mediated the association of sleep disturbance and functional difficulties related to OA (Supplementary Data).
Discussion
In a sample of African American and white US veterans with knee OA, we sought to determine if pain catastrophizing and arthritis self-efficacy were cross-sectional mediators of the association of sleep disturbance and overall OA symptom severity. We also examined whether these pain-related beliefs played an intermediary role linking sleep disturbance with specific symptoms (i.e., pain severity and physical functioning). We found that more frequent sleep disturbance was associated with higher levels of OA symptom severity both directly and indirectly, through higher levels of pain catastrophizing and lower levels of arthritis self-efficacy. A similar pattern of findings emerged when we specifically examined pain severity and functioning as outcomes.
Our findings both complement and extend existing literature investigating sleep and pain. The direct positive association of sleep disturbance with OA symptom severity observed in this study corroborates existing work relating poor sleep to heightened pain experiences and worse self-reported physical functioning [2,3,5]. Our analysis also adds to the limited number of studies that have investigated the interrelations of sleep, pain catastrophizing, and pain [9,41] by examining differing conceptualizations of how these processes relate, with the additional consideration of the role of self-efficacy as an adaptive pain-related cognition. Our findings that sleep disturbance was related to OA symptom severity, pain severity, and self-reported physical function indirectly through pain catastrophizing and self-efficacy are novel and align with the conceptualization of pain as a multifactorial phenomenon linked to sleep via multiple pathways, including pain-related cognitions [2,7].
Though individuals’ inclinations toward catastrophizing and sense of self-efficacy in the context of pain may be dispositional to some extent, they can also be influenced by situation-specific factors and are modifiable through intervention [42–44]. Results from the current analysis suggest that sleep is among the situational factors relevant to pain catastrophizing and pain self-efficacy. The mechanisms underlying the associations of sleep with pain catastrophizing and pain self-efficacy have yet to be clarified. However, previous research has linked sleep disturbance and sleep deprivation to worse cognitive [45,46] and affective functioning [47–49], suggesting the explanatory potential of cognitive and affective processes.
Due to the cross-sectional nature of the data used in the current analysis and existing evidence for other configurations of the variables of interest [9,41], we assessed an alternative multiple mediation model wherein OA symptom severity was the primary predictor and sleep disturbance was the outcome. In this model, greater OA symptom severity was significantly associated with more frequent sleep disturbance directly and indirectly only through pain catastrophizing; arthritis self-efficacy was not a significant pathway linking OA symptom severity and sleep disturbance. This pattern of findings aligns with Vitiello and colleagues’ proposition that sleep, pain catastrophizing, and pain are linked via a positive feedback loop [7]. However, the same may not be true for self-efficacy. Although temporality cannot be established using cross-sectional data, the finding that sleep disturbance was associated with pain self-efficacy when examined as predictor but not an outcome suggests that sleep disturbance may be more likely to negatively impact self-efficacy than the reverse (low levels of self-efficacy negatively impacting sleep disturbance).
If replicated in studies with causal research designs, our findings have several implications for the management of pain. The primary findings suggest the potential value of incorporating strategies targeting sleep and pain-related beliefs, both adaptive and maladaptive, to promote effective pain management in persons with chronic pain. Findings from the alternative explanatory model suggest that interventions targeting OA symptom severity alone may similarly be inadequate to affect sleep and that intervention on pain catastrophizing may bolster treatment effects. The application of multicomponent, nonpharmacological interventions targeting pain and insomnia has been of growing interest, with promising findings in a limited number of studies [50–53]. Our findings reinforce the need for further clinical studies of treatments that address both pain-related beliefs and sleep disturbance in individuals with osteoarthritis.
Limitations of the current study are worth noting. Foremost, causal inferences cannot be drawn due to the observational, cross-sectional study design. However, experimental and prospective research supports the current conceptualization of sleep disturbance, pain catastrophizing and pain self-efficacy, and pain severity [2,6,18]. Recent reviews of prospective studies of sleep and pain have concluded that sleep disturbance and sleep deterioration exacerbate existing pain conditions and heighten the risk of new onset of pain conditions [2,6]. Though more nascent, limited research has also favored sleep disturbance as a predictor of pain catastrophizing but not the reverse [18]. Replication of the current conceptualization of sleep, pain catastrophizing, pain self-efficacy, and pain using microlongitudinal (e.g., daily measurement of the variables of interest, over multiple days to weeks) or experimental study designs would provide additional clarification for how these phenomena interrelate.
An additional limitation is that sleep disturbance was measured retrospectively using a single self-report item. Consequently, information regarding specific sleep parameters was not gleaned, and the measure was susceptible to potential biases encountered in self-report measures more generally. Additionally, we did not collect data on the use of pharmacological or nonpharmacological methods for managing sleep disorders as part of this study and thus were unable to account for this in our analyses. Nevertheless, the average frequency of sleep disturbance in the current sample is comparable to the average PHQ-assessed sleep disturbance reported in Koffel and colleagues’ recent evaluation of sleep and pain in adult veterans (aged 19–65 years) with chronic musculoskeletal pain [5]. It is also consistent, more generally, with literature indicating that sleep disturbance frequently co-occurs with arthritis [37,38]. The prevalence of EHR-derived insomnia diagnoses was approximately 9% in this sample. This rate is lower than estimates derived from self-report measures of insomnia in veterans with chronic pain [54] but is consistent with work conducted using VA EHRs [55,56]. The lower rate of insomnia diagnoses using this method may be a reflection of the underdetection of insomnia in routine clinical care [57]. It may also reflect under-reporting of diagnoses in the VA EHRs, specifically, relative to non-VA health care settings that rely on diagnostic codes for billing purposes [55,58,59].
Conclusions
In summary, this cross-sectional analysis identified that sleep and pain-related experiences are related directly and indirectly, through pain catastrophizing and self-efficacy, in veterans with pain from knee OA. These findings lend empirical support to theoretical models suggesting that pain-related cognitions may be one of the numerous biobehavioral processes underlying the association of sleep and pain [2,7,44].
Supplementary Data
Supplementary data are available at Pain Medicine online.
Supplementary Material
Funding sources: This work was supported by the Veterans Health Administration Health Services Research and Development Service (IIR13-080; Principal Investigator: Dr. Hausmann). Dr. Tighe is supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment and the VISN 4 Mental Illness Research, Education and Clinical Center (Director: D. Oslin; Pittsburgh Site Director: G. Haas), VA Pittsburgh Healthcare System.
Disclaimer: The contents of this work do not represent the views of the Department of Veterans Affairs, Department of Defense, or the United States Government.
Disclosure and conflicts of interest: Ernest Vina discloses his role as site Principal Investigator in a cohort study sponsored by Astrazeneca. C. Kent Kwoh discloses institutional grants from AbbVie and EMD Serono, consultant and data safety monitoring board roles with Astellas, and consultant and advisory board roles with EMD Serono and Fidia. Caitlan Tighe, Ada Youk, Said Ibrahim, Debra Weiner, Rollin Gallagher, Adam Bramoweth, and Leslie Hausmann have no conflicts to disclose.
Copyright statement: Because several of the authors of this manuscript are employees of the US Government and contributed to this manuscript as part of their official duties, the work is not subject to US copyright.
References
- 1.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC.. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol Bull 2007;133(4):581–624. [DOI] [PubMed] [Google Scholar]
- 2.Finan PH, Goodin BR, Smith MT.. The association of sleep and pain: An update and a path forward. J Pain 2013;14(12):1539–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith MT, Haythornthwaite JA.. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev 2004;8(2):119–32. [DOI] [PubMed] [Google Scholar]
- 4.Lautenbacher S, Kundermann B, Krieg JC.. Sleep deprivation and pain perception. Sleep Med Rev 2006;10(5):357–69. [DOI] [PubMed] [Google Scholar]
- 5.Koffel E, Kroenke K, Bair MJ, Leverty D, Polusny MA, Krebs EE.. The bidirectional relationship between sleep complaints and pain: Analysis of data from a randomized trial. Health Psychol 2016;35(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Afolalu EF, Ramlee F, Tang N.. Effects of sleep changes on pain-related health outcomes in the general population: A systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev 2018;39:82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ.. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med 2009;5(4):355–62. [PMC free article] [PubMed] [Google Scholar]
- 8.Byers HD, Lichstein KL, Thorn BE.. Cognitive processes in comorbid poor sleep and chronic pain. J Behav Med 2016;39(2):233–40. [DOI] [PubMed] [Google Scholar]
- 9.Buenaver LF, Quartana PJ, Grace EG.. Evidence for indirect effects of pain catastrophizing on clinical pain among myofascial temporomandibular disorder participants: The mediating role of sleep disturbance. Pain 2012;153(6):1159–66. [DOI] [PubMed] [Google Scholar]
- 10.Jackson T, Wang Y, Wang Y, Fan H.. Self-efficacy and chronic pain outcomes: A meta-analytic review. J Pain 2014;15(8):800–14. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan MJL, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain 2001;17(1):52–64. [DOI] [PubMed] [Google Scholar]
- 12.Marks R.Self-efficacy and arthritis disability: An updated synthesis of the evidence base and its relevance to optimal patient care. Health Psychol Open 2014;1(1):205510291456458.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Calderon J, Zamora-Campos C, Navarro-Ledesma S, Luque-Suarez A.. The role of self-efficacy on the prognosis of chronic musculoskeletal pain: A systematic review. J Pain 2018;19(1):10–34. [DOI] [PubMed] [Google Scholar]
- 14.Roberts MB, Drummond PD.. Sleep problems are associated with chronic pain over and above mutual associations with depression and catastrophizing. Clin J Pain 2016;32(9):792–9. [DOI] [PubMed] [Google Scholar]
- 15.Campbell CM, Buenaver LF, Finan P, et al. Sleep, pain catastrophizing, and central sensitization in knee osteoarthritis patients with and without insomnia. Arthritis Care Res (Hoboken) 2015;67(10):1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerhart JI, Burns JW, Post KM, et al. Relationships between sleep quality and pain-related factors for people with chronic low back pain: Tests of reciprocal and time of day effects. Ann Behav Med 2017;51(3):365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lami MJ, Martínez MP, Sánchez AI, et al. Gender differences in patients with fibromyalgia undergoing cognitive-behavioral therapy for insomnia: Preliminary data. Pain Pract 2016;16(2):E23–34. [DOI] [PubMed] [Google Scholar]
- 18.Lerman SF, Finan PH, Smith MT, Haythornthwaite JA.. Psychological interventions that target sleep reduce pain catastrophizing in knee osteoarthritis. Pain 2017;158(11):2189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez MP, Miró E, Sánchez AI, et al. Cognitive-behavioral therapy for insomnia and sleep hygiene in fibromyalgia: A randomized controlled trial. J Behav Med 2014;37(4):683–97. [DOI] [PubMed] [Google Scholar]
- 20.Smith MT, Finan PH, Buenaver LF, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: A randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol 2015;67(5):1221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturgeon JA, Zautra AJ.. Resilience: A new paradigm for adaptation to chronic pain. Curr Pain Headache Rep 2010;14(2):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong AD, Kim S, Young S, Steptoe A.. Positive affect and sleep: A systematic review. Sleep Med Rev 2017;35:21–32. [DOI] [PubMed] [Google Scholar]
- 23.Scullin MK, Bliwise DL.. Sleep, cognition, and normal aging: Integrating a half century of multidisciplinary research. Perspect Psychol Sci 2015;10(1):97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liedberg GM, Björk M, Börsbo B.. Self-reported nonrestorative sleep in fibromyalgia-relationship to impairments of body functions, personal function factors, and quality of life. J Pain Res 2015;8:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miró E, Martínez MP, Sánchez AI, Prados G, Medina A.. When is pain related to emotional distress and daily functioning in fibromyalgia syndrome? The mediating roles of self-efficacy and sleep quality. Br J Health Psychol 2011;16(4):799–814. [DOI] [PubMed] [Google Scholar]
- 26.Hausmann LRM, Ibrahim SA, Kwoh CK, et al. Rationale and design of the Staying Positive with Arthritis (SPA) Study: A randomized controlled trial testing the impact of a positive psychology intervention on racial disparities in pain. Contemp Clin Trials 2018;64:243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hausmann LM, Youk A, Kwoh C, et al. Effect of a positive psychological intervention on pain and functional difficulty among adults with osteoarthritis: A randomized clinical trial. JAMA Netw Open 2018;1(5):e182533.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nevitt M, Felson D, Lester G.. Protocol for the Cohort Study. The Osteoarthritis Initiative; 2006. http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf (accessed July 24, 2019). [Google Scholar]
- 29.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC.. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care 2002;40(9):771–81. [DOI] [PubMed] [Google Scholar]
- 30.Kroenke K, Spitzer RL, Williams JB.. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacGregor KL, Funderburk JS, Pigeon W, Maisto SA.. Evaluation of the PHQ-9 item 3 as a screen for sleep disturbance in primary care. J Gen Intern Med 2012;27(3):339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bastien CH, Vallières A, Morin CM.. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 33.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW.. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15(12):1833–40. [PubMed] [Google Scholar]
- 34.Sullivan MJ, Bishop SR, Pivik J.. The Pain Catastrophizing Scale: Development and validation. Psychol Assess 1995;7(4):524–32. [Google Scholar]
- 35.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR.. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum 1989;32(1):37–44. [DOI] [PubMed] [Google Scholar]
- 36.Gaines JM, Talbot LA, Metter EJ.. The relationship of arthritis self-efficacy to functional performance in older men and women with osteoarthritis of the knee. Geriatr Nurs 2002;23(3):167–70. [DOI] [PubMed] [Google Scholar]
- 37.Parker JC, Smarr KL, Buckelew SP, et al. Effects of stress management on clinical outcomes in rheumatoid arthritis. Arthritis Rheum 1995;38(12):1807–18. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire. Atlanta: Centers for Disease Control and Prevention; 2010. http://www.cdc.gov/brfss/questionnaires/pdf-ques/2010brfss.pdf (accessed July 24, 2019). [Google Scholar]
- 39.Cohen J.Statistical Power Analysis for the Behavioral Sciences. 2nd ed.Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 40.Hayes AF.Introduction to Mediation, Moderation, and Conditional Process Analysis a Regression-Based Approach. New York: Guilford Press; 2013. [Google Scholar]
- 41.Wilt JA, Davin S, Scheman J.. A multilevel path model analysis of the relations between sleep, pain, and pain catastrophizing in chronic pain rehabilitation patients. Scand J Pain 2016;10(1):122–9. [DOI] [PubMed] [Google Scholar]
- 42.Schütze R, Rees C, Smith A, Slater H, Campbell JM, O'Sullivan P.. How can we best reduce pain catastrophizing in adults with chronic noncancer pain? A systematic review and meta-analysis. J Pain 2018;19(3):233–56. [DOI] [PubMed] [Google Scholar]
- 43.Turner JA, Holtzman S, Mancl L.. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain 2007;127(3):276–86. [DOI] [PubMed] [Google Scholar]
- 44.Marks R.Self-efficacy and its application in the treatment of knee osteoarthritis: A critical review. Rheum Rep 2012;4(1):34–45. [Google Scholar]
- 45.Lo JC, Groeger JA, Cheng GH, Dijk DJ, Chee MW.. Self-reported sleep duration and cognitive performance in older adults: A systematic review and meta-analysis. Sleep Med 2016;17:87–98. [DOI] [PubMed] [Google Scholar]
- 46.Fortier-Brochu E, Beaulieu-Bonneau S, Ivers H, Morin CM.. Insomnia and daytime cognitive performance: A meta-analysis. Sleep Med Rev 2012;16(1):83–94. [DOI] [PubMed] [Google Scholar]
- 47.Tempesta D, Socci V, De Gennaro L, Ferrara M.. Sleep and emotional processing. Sleep Med Rev 2018;40:183–95. [DOI] [PubMed] [Google Scholar]
- 48.Kahn M, Sheppes G, Sadeh A.. Sleep and emotions: Bidirectional links and underlying mechanisms. Int J Psychophysiol 2013;89(2):218–28. [DOI] [PubMed] [Google Scholar]
- 49.Konjarski M, Murray G, Lee VV, Jackson ML.. Reciprocal relationships between daily sleep and mood: A systematic review of naturalistic prospective studies. Sleep Med Rev 2018;42:47–58. [DOI] [PubMed] [Google Scholar]
- 50.Tang NK, Goodchild CE, Salkovskis PM.. Hybrid cognitive-behaviour therapy for individuals with insomnia and chronic pain: A pilot randomised controlled trial. Behav Res Ther 2012;50(12):814–21. [DOI] [PubMed] [Google Scholar]
- 51.Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: The lifestyles randomized controlled trial. J Am Geriatr Soc 2013;61(6):947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vitiello MV, McCurry SM, Shortreed SM, et al. Short-term improvement in insomnia symptoms predicts long-term improvements in sleep, pain, and fatigue in older adults with comorbid osteoarthritis and insomnia. Pain 2014;155(8):1547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCurry SM, Shortreed SM, Von Korff M, et al. Who benefits from CBT for insomnia in primary care? Important patient selection and trial design lessons from longitudinal results of the lifestyles trial. Sleep 2014;37(2):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor SS, Hughes JM, Coffman CJ, et al. Prevalence of and characteristics associated with insomnia and obstructive sleep apnea among veterans with knee and hip osteoarthritis. BMC Musculoskelet Disord 2018;19(1):79. doi: 10.1186/s12891-018-1993-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hermes E, Rosenheck R.. Prevalence, pharmacotherapy and clinical correlates of diagnosed insomnia among Veterans Health Administration service users nationally. Sleep Med 2014;15(5):508–14. [DOI] [PubMed] [Google Scholar]
- 56.Alexander M, Ray MA, Hébert JR, et al. The National Veteran Sleep Disorder Study: Descriptive epidemiology and secular trends, 2000–2010. Sleep 2016;39(7):1399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulmer CS, Bosworth HB, Beckham JC, et al. Veterans Affairs primary care provider perceptions of insomnia treatment. J Clin Sleep Med 2017;13(08):991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosen AK, Gardner J, Montez M, Loveland S, Hendricks A.. Dual-system use: Are there implications for risk adjustment and quality assessment? Am J Med Qual 2005;20(4):182–94. [DOI] [PubMed] [Google Scholar]
- 59.Byrne MM, Kuebeler M, Pietz K, Petersen LA.. Effect of using information from only one system for dually eligible health care users. Med Care 2006;44(8):768–73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.