Figure 2.
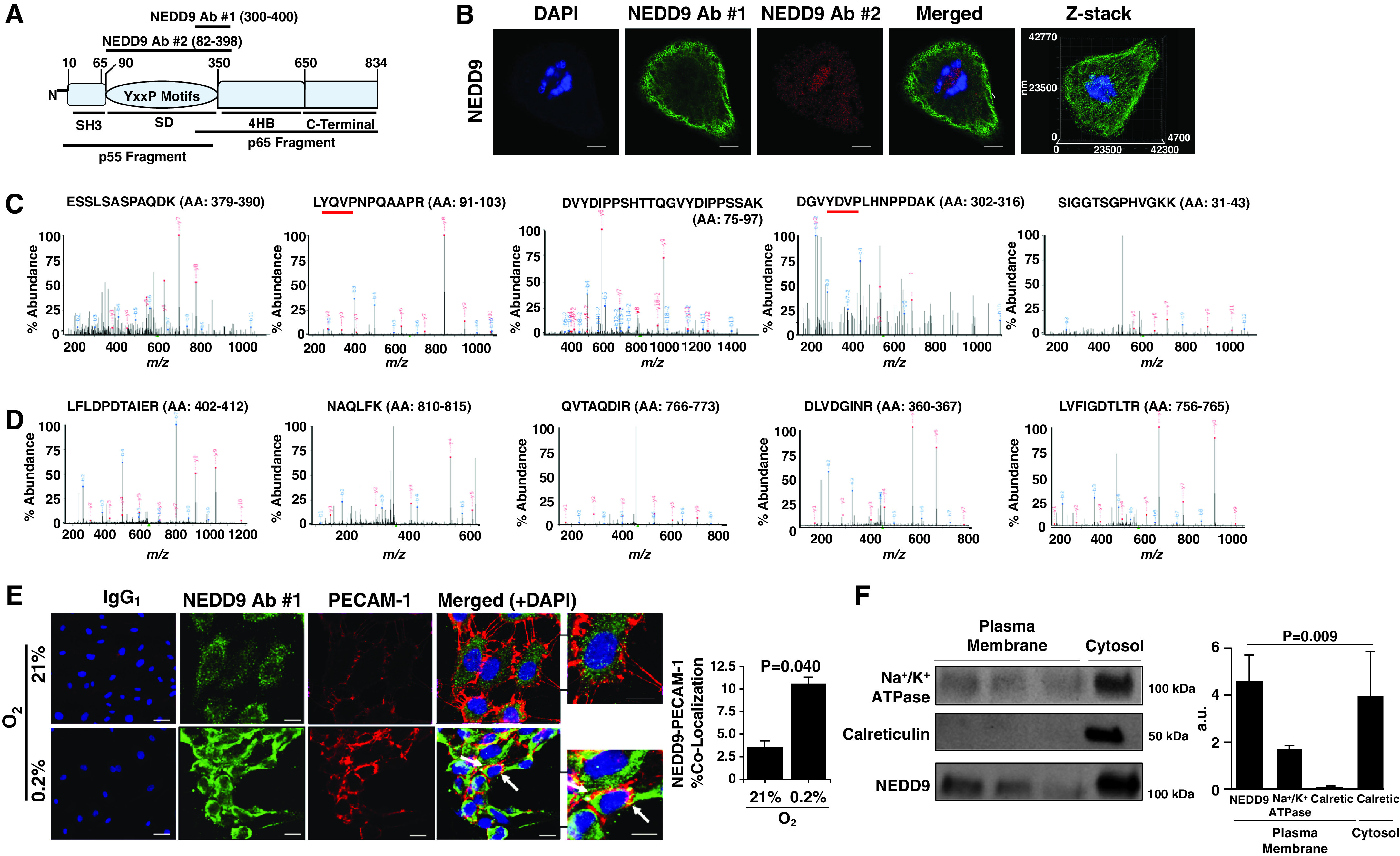
The NEDD9 substrate domain is expressed on the extracellular plasma membrane of human pulmonary endothelial cells (HPAECs). (A) NEDD9 is a scaffolding protein and in Homo sapiens is composed of 834 amino acids organized in four distinct domains: the SH3, substrate, 4HB, and C-terminal domains. Two NEDD9 cleavage peptide fragments (p55 kD and p65 kD) have been reported previously (22). To determine whether either cleavage product corresponded to differences in NEDD9 localization in HPAECs, anti-NEDD9 immunofluorescence was performed using NEDD9 antibody (Ab) 1 targeting the p55-kD fragment and NEDD9 Ab 2 targeting the p65-kD fragment. (B) Compared with NEDD9 Ab 2, NEDD9 expression detected using NEDD9 Ab 1 was localized predominantly to the perimeter of cells (n = 3). Scale bar = 10 μm. (C) The MS spectra from five abundant peptides detected in trypsin-digested HPAEC lysates immunoprecipitated using NEDD9 Ab 1 corresponded exclusively to the p55-kD fragment, whereas (D) NEDD9 Ab 2 identified NEDD9 peptides corresponding to the p65-kD fragment (n = 3). The red underlining denotes a tyrosine-x-x-proline (YxxP) sequence, where x is another amino acid. (E) Compared with normoxia, hypoxia (0.2% O2) for 24 hours increased colocalization of NEDD9 with the endothelial plasma-membrane protein PECAM-1 (platelet–endothelial cell adhesion molecule 1) analyzed using double immunofluorescence (n = 3) (colocalization indicated by white arrow). IgG1 represents the control. (F) Isolation of HPAEC plasma-membrane fractions was confirmed by Na+/K+ ATPase expression in the absence of (cytosolic) calreticulin, and NEDD9 was analyzed in the plasma-membrane fraction by immunoblotting (n = 5). Scale bar, 40 μm. Data are presented as the mean ± SE. Representative immunoblots and micrographs are presented. a.u. = arbitrary units; Calciretic = calreticulin; MS = mass spectrometry; m/z = mass-to-charge ratio.
