Abstract
Rationale: Current diagnostic tests fail to identify individuals at higher risk of progression to tuberculosis disease, such as those with recent Mycobacterium tuberculosis infection, who should be prioritized for targeted preventive treatment.
Objectives: To define a blood-based biomarker, measured with a simple flow cytometry assay, that can stratify different stages of tuberculosis infection to infer risk of disease.
Methods: South African adolescents were serially tested with QuantiFERON-TB Gold to define recent (QuantiFERON-TB conversion <6 mo) and persistent (QuantiFERON-TB+ for >1 yr) infection. We defined the ΔHLA-DR median fluorescence intensity biomarker as the difference in HLA-DR expression between IFN-γ+ TNF+ Mycobacterium tuberculosis–specific T cells and total CD3+ T cells. Biomarker performance was assessed by blinded prediction in untouched test cohorts with recent versus persistent infection or tuberculosis disease and by unblinded analysis of asymptomatic adolescents with tuberculosis infection who remained healthy (nonprogressors) or who progressed to microbiologically confirmed disease (progressors).
Measurements and Main Results: In the test cohorts, frequencies of Mycobacterium tuberculosis–specific T cells differentiated between QuantiFERON-TB− (n = 25) and QuantiFERON-TB+ (n = 47) individuals (area under the receiver operating characteristic curve, 0.94; 95% confidence interval, 0.87–1.00). ΔHLA-DR significantly discriminated between recent (n = 20) and persistent (n = 22) QuantiFERON-TB+ (0.91; 0.83–1.00); persistent QuantiFERON-TB+ and newly diagnosed tuberculosis (n = 19; 0.99; 0.96–1.00); and tuberculosis progressors (n = 22) and nonprogressors (n = 34; 0.75; 0.63–0.87). However, ΔHLA-DR median fluorescent intensity could not discriminate between recent QuantiFERON-TB+ and tuberculosis (0.67; 0.50–0.84).
Conclusions: The ΔHLA-DR biomarker can identify individuals with recent QuantiFERON-TB conversion and those with disease progression, allowing targeted provision of preventive treatment to those at highest risk of tuberculosis. Further validation studies of this novel immune biomarker in various settings and populations at risk are warranted.
Keywords: tuberculosis infection, QuantiFERON-TB Gold, biomarker, tuberculosis risk, recent tuberculosis infection
At a Glance Commentary
Scientific Knowledge on the Subject
Preventive treatment of individuals with Mycobacterium tuberculosis (M.tb) infection who are at high risk of progressing to active tuberculosis is a key strategy to reduce the global tuberculosis burden. Current diagnostic tests can identify persons with tuberculosis and those with M.tb infection; however, these tests cannot distinguish who, among those with asymptomatic M.tb infection, are most likely to progress to tuberculosis and would therefore benefit most from preventive treatment.
What This Study Adds to the Field
We present novel evidence that M.tb-specific T cells from healthy individuals with recently acquired M.tb infection and individuals progressing to active disease are highly activated but M.tb-specific T cells from those with remote M.tb infection and nonprogressors are not. The ΔHLA-DR median fluorescent intensity biomarker has utility as a screening test to identify individuals who would warrant further clinical investigation and microbiological tests to guide the administration of either tuberculosis preventive therapy or a full course of tuberculosis treatment. Further large-scale validation of the ΔHLA-DR median fluorescent intensity biomarker is warranted.
Tuberculosis (TB) is an ongoing global threat causing an estimated 10 million incident cases and approximately 1.4 million deaths (1) and was the leading cause of death due to a single infectious agent in 2019. A key strategy to alleviate the global burden of TB, promoted by the World Health Organization, is to provide TB preventive therapy (TPT) to individuals with Mycobacterium tuberculosis (M.tb) infection who are at risk of disease progression (1, 2). Current diagnostic tests can identify persons with TB (sputum-based microbiological tests) and those with infection (tuberculin skin test [TST] or IFN-γ release assays [IGRAs]). However, these tests cannot distinguish who, among those with asymptomatic infection, are at high risk of progressing to active disease and should receive TPT.
In countries with low TB burden and/or high income, TPT is standard of care for persons with clinical and epidemiological risk factors or a diagnosis of M.tb infection, and it reduces the risk of TB (3, 4). On the other hand, in many countries where M.tb is endemic, >50% of adults may already be infected (5), making the provision of TPT infeasible and unaffordable, especially without knowledge of exposure history and considering the high risk of reinfection after treatment completion in high transmission settings (6, 7). Furthermore, given the small proportion of individuals with M.tb infection who are actually at risk of TB progression, the provision of TPT to all those infected exposes many individuals to unnecessary side effects.
The highest risk of TB progression occurs within the first 1–2 years after primary infection (8–10). By contrast, more remote or persistent infection is associated with a lower risk of TB (8, 9). In fact, remote M.tb infection has been associated with a significantly lower risk of disease progression compared with recent infection (11, 12), and there is evidence that many individuals with remote M.tb exposure may actually have cleared M.tb (13). A blood-based biomarker that could distinguish between recent and remote infection would therefore allow targeted TPT to those with recent infection and could potentially transform the clinical management of TB, regardless of setting (14). Unfortunately, current diagnostic tests for M.tb infection only measure immunological sensitization to M.tb and cannot distinguish between recent and remote infection. Improving tools for detecting M.tb infection and testing for progression to TB is a key priority in the Global Plan to End TB (15).
M.tb-specific T-cell features, such as activation (16–20), differentiation (21, 22) and cytokine coexpression profiles (23, 24), have shown promising diagnostic applications to distinguish M.tb infection from TB, and to monitor treatment response. The underlying hypothesis is that all these biomarkers reflect M.tb antigen load, which is higher during TB compared with controlled infection, and that treatment effectively reduces M.tb load (16, 20). We postulated that recent M.tb infection is associated with a high initial M.tb load that is ultimately controlled by immune responses in those with remote infection who do not progress to disease. We also postulated that progression from M.tb infection to disease is associated with an increase in M.tb load (25). This is supported by our previous study in progressors, which showed increased inflammation and activation of bulk CD4 T cells before clinical onset (26). In line with this principle, Halliday and colleagues discovered that proportions of M.tb-specific TNF+ IFN-γ− IL-2− CD4 T cells with an effector phenotype distinguished between recent and remote M.tb infection (27).
In this study, we hypothesized that individuals with high-risk M.tb infection, associated with recent QuantiFERON-TB Gold (QFT) conversion, and progressors to TB disease have higher levels of M.tb-specific T-cell activation in peripheral blood compared with individuals with persistently QFT-positive results at low risk of progression. We aimed to develop a simplified assay that could stratify, for the first time, the entire spectrum of M.tb from acquisition of infection to clinical disease and through treatment response that is amenable to clinical translation and further large-scale clinical validation.
Some of the results of these studies have been previously reported in the form of abstracts (28–33) and a preprint (medRxiv [29 June 2020] https://doi.org/10.1101/2020.06.26.20135665).
Methods
Study Participants
Different groups of adolescents and adults enrolled in observational studies approved by the University of Cape Town Human Research Ethics Committee (protocol references: 045/2005, 088/2008, and 102/2017) were included the training, test, and TB progressor cohorts (Figure 1 and Table 1). Detailed inclusion and exclusion criteria are described in the online supplement.
Figure 1.
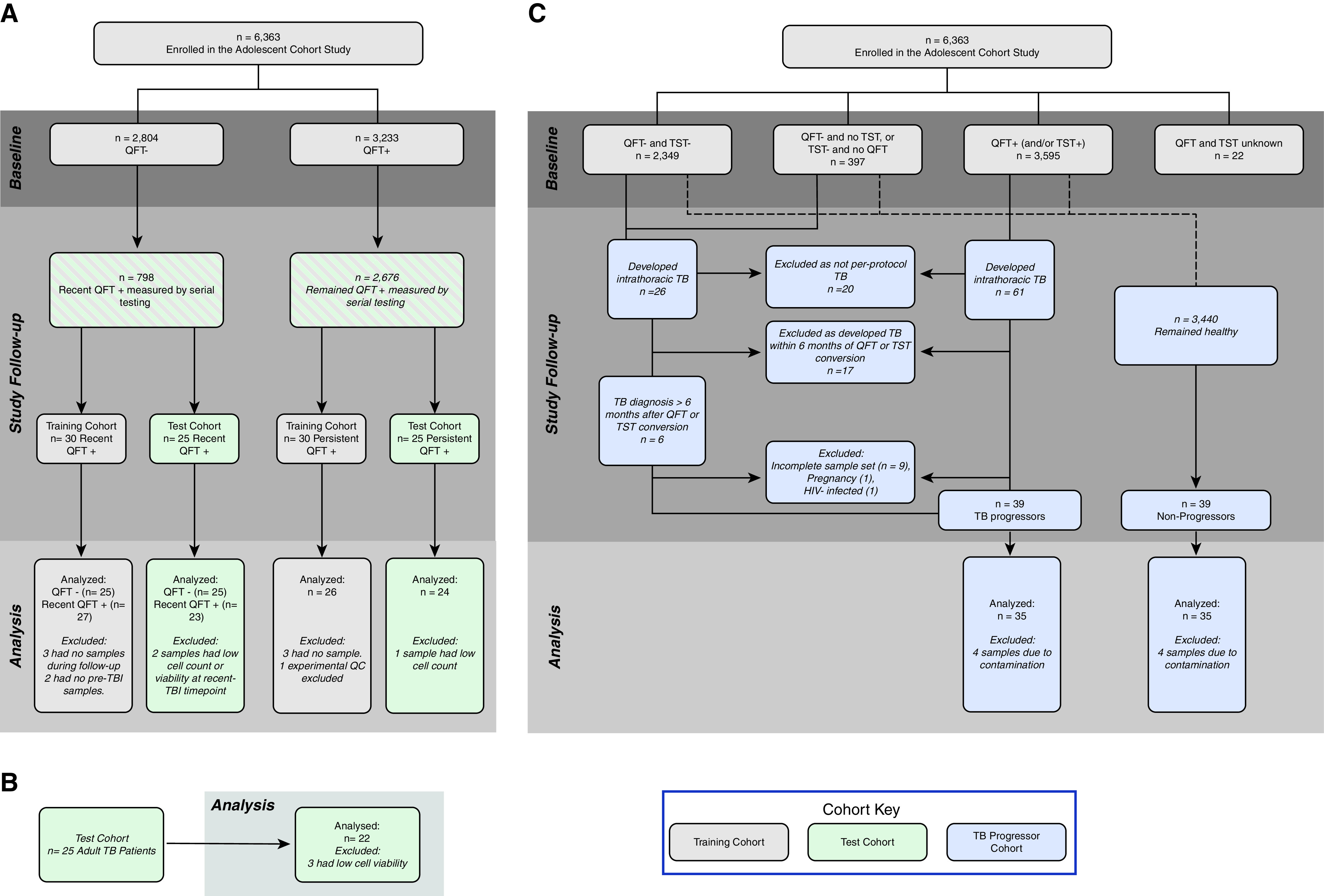
Study design. Adolescent participants were selected on the basis of peripheral blood mononuclear cell sample availability from a larger epidemiological study, the Adolescent Cohort Study (10). (A) Inclusions and exclusions for recent and remote QFT+ individuals in the training and test cohorts. (B) Adults with tuberculosis diagnosis sampled cross-sectionally (40). (C) Reasons for inclusion and exclusion of participants in progressor and nonprogressor cohorts. QC = quality control; QFT = QuantiFERON-TB Gold; TB = tuberculosis; TBI = tuberculosis infection; TST = tuberculin skin test.
Table 1.
Demographics
| Training Cohort |
Test Cohort |
TB Progressor Cohort |
|||||
|---|---|---|---|---|---|---|---|
| Persistent QFT+ | Recent QFT+ | Persistent QFT+ | Recent QFT+ | TB | Progressors | Nonprogressors | |
| n | 30 | 30 | 25 | 25 | 25 | 39 | 39 |
| Sex, F | 21 | 19 | 12 | 9 | 3 | 14 | 28 |
| Age, median (IQR), yr | 15 (14–16) | 15 (14–16) | 15 (14–16) | 15 (14–16) | 32 (21–42) | 16 (15–17) | 16 (14–17) |
| Ethnicity: colored, n | 26 | 29 | 23 | 23 | 14 | 36 | 37 |
| Ethnicity: Black, n | 4 | 1 | 2 | 1 | 11 | 3 | 2 |
| Ethnicity: Indian, n | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
Definition of abbreviations: IQR = interquartile range; QFT = QuantiFERON-TB Gold In-Tube; TB = tuberculosis.
Adolescents were selected on the basis of QFT (Cellestis) and/or TST results and sample availability from a large epidemiological study conducted in the Worcester Area, Western Cape, South Africa (2005–2009), a region with high TB incidence (10). Recent M.tb infection (recent QFT+) was defined in healthy participants by two negative QFT tests (IFN-γ <0.35 IU/ml with at least one test <0.2 IU/ml) followed by two positive QFT tests (IFN-γ ≥0.35 IU/ml with at least one test >0.7 IU/ml) 6 months apart over 1.5 years (Figures E1A and E1B in the online supplement) (34). Remote M.tb infection (persistent QFT+) was defined in healthy participants by four consecutive positive QFT tests 6 months apart over 1.5 years with at least two QFT-positive results >0.7 IU/ml (Figures E1A and E1B). Clinical definitions for the adolescents selected for TB nonprogressors and progressors in the TB progressor cohort have been described (26, 35) and are available in the online supplement.
Adults with recently diagnosed pulmonary TB by positive Xpert MTB/RIF (Cepheid) were recruited before treatment initiation.
Peripheral Blood Mononuclear Cell Stimulation and Staining Protocols
Cryopreserved peripheral blood mononuclear cells (PBMCs) were thawed and stimulated with peptide pools spanning the full length of CFP-10 (10-kDa culture filtrate protein)/ESAT-6 (6-kDa early secretory antigenic target), antigens used in the QFT assay, for 18 hours (training and test cohorts) or M.tb lysate (H37Rv) for 12 hours (TB progressor cohort). Cells were stained and analyzed by flow cytometry (Tables E1–E3). M.tb-specific cells were defined by cytokine expression, detected either by intracellular cytokine staining (ICS; test and training cohorts), or gene expression from sorted single CD69+ CD137+ and/or CD69+ CD154+ T cells (TB progressor cohort). Different stimulation and detection protocols were used because data from the TB progressor cohort were from an unpublished study that aimed to perform single-cell T-cell receptor sequencing and measurement of selected transcripts. CD3 and HLA-DR expression were measured by flow cytometry, whereas IFNG and TNF mRNA expression in sorted single M.tb-specific cells were quantified by RNA sequencing using Illumina MiSeq, as previously described (36, 37). Detailed information about protocols used is provided in the online supplement.
Data Analysis
Responder definition
In the training and test cohorts, we identified responders as individuals with relative counts of IFN-γ+ TNF+ CD3+ T cells in the antigen-stimulated condition that were significantly higher (P ≤ 0.01 by Fisher exact test) than in the unstimulated condition and had a fold change of antigen-specific T-cell frequencies of three or more. In the TB progressor cohort, only samples with 10 or more IFNG and TNF coexpressing cells were analyzed for HLA-DR expression (see below).
T-cell activation biomarker definition
In the training and TB progressor cohorts, we explored different calculations to define the T-cell activation biomarker: HLA-DR+ T cells as a percentage of total IFN-γ+ TNF+ T cells, HLA-DR median fluorescent intensity (MFI) ratio (Equation 1) and ΔHLA-DR MFI (Equation 2, Figure 2D).
| (1) |
| (2) |
Figure 2.
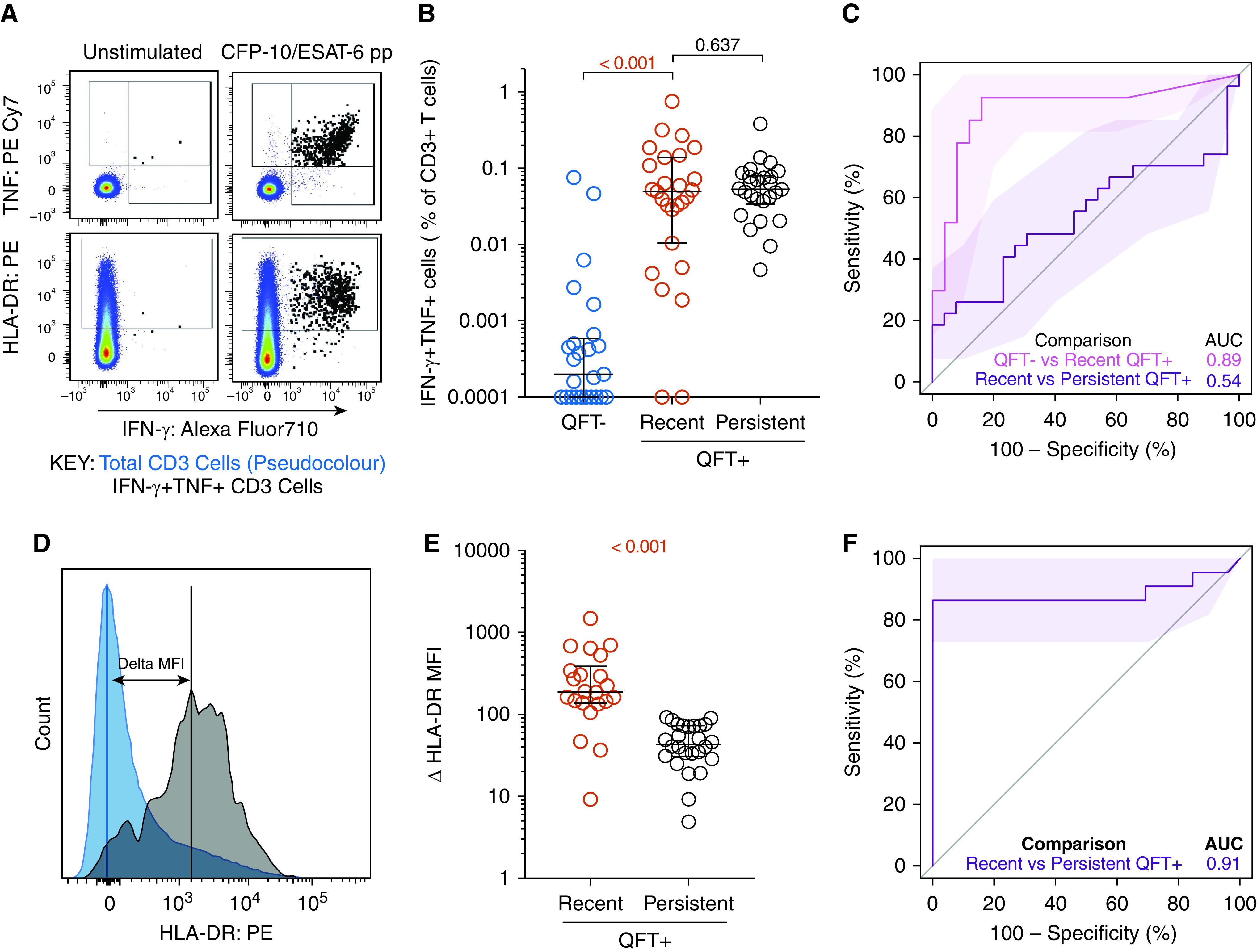
Recent QuantiFERON-TB Gold (QFT)+ conversion is associated with higher T-cell activation than persistent QFT+ results. (A) Representative flow cytometry plots depicting IFN-γ, TNF, and HLA-DR expression in CD3+ T cells. IFN-γ+ TNF+ and total CD3+ T cells are depicted by black and pseudocolor dots, respectively. (B) Frequencies of background subtracted CFP-10/ESAT-6–specific IFN-γ+ TNF+ CD3+ T cells detected before (QFT−; blue; n = 25) and after (recent QFT+; red; n = 27) infection, and in persistent QFT+ (black; n = 26) individuals in the training cohort. (C) Area under the receiver operating characteristic curve (AUROC) showing performance of CFP-10/ESAT-6–specific IFN-γ+ TNF+ CD3+ T cells to discriminate between QFT− and recent QFT+ individuals and between recent and persistent QFT+ individuals. (D) Representative flow cytometry histogram overlay of HLA-DR expression levels by IFN-γ+ TNF+ CD3+ T cells (black) and total CD3+ T cells (blue) and how ΔHLA-DR median fluorescent intensity (MFI) is calculated. (E) ΔHLA-DR MFI in recent (n = 22) and persistent (n = 26) QFT+ responders in the training cohort. (F) Performance of ΔHLA-DR MFI to discriminate between recent and persistent QFT+ individuals. P values were calculated using the Wilcoxon signed-rank test for paired (QFT− vs. recent QFT+) or Mann-Whitney U test for unpaired (recent vs. persistent QFT+) comparisons. Where appropriate, we corrected for multiple comparison as described in the Methods. P values highlighted in red are considered significant. Shaded areas in AUROC plots depict 95% confidence intervals. Values <0.0001 were set to 0.0001 to allow display on a logarithmic scale. AUC = area under the curve; pp = peptide pool.
Statistical Analysis
Statistical analyses were performed using R and GraphPad Prism v7. We performed Wilcoxon signed-rank and Mann-Whitney U tests for paired and unpaired analyses, respectively. We corrected for multiple comparisons using the Bonferroni method as appropriate for the numbers of groups compared for each hypothesis/experiment. Biomarker selection was performed in the training cohort and was tested in a blinded fashion in the test cohort. Receiver operating characteristic (ROC) curve analysis and selection of the ΔHLA-DR MFI threshold were performed using the pROC package in R (version 1.15.3; https://web.expasy.org/pROC/) (38). We selected the ΔHLA-DR MFI threshold for the test cohort using persistent QFT+ and TB groups and then applied the same threshold to compare the performance of the biomarker between recent and persistent QFT+. The ΔHLA-DR MFI threshold for the TB progressor cohort was defined independently.
Results
To identify potential biomarkers of recent M.tb infection, we first evaluated a training cohort of adolescents with recent (n = 30) and persistent (n = 30) QFT+ responses (Figure 1A and Table 1) and compared T-cell polyfunctional, memory, and activation profiles between the two M.tb infection states. Results from these comprehensive immunological analyses, which inform immune responses and biological pathways associated with recent and remote infection, are presented elsewhere (39). Here we focused on developing a single candidate biomarker that distinguished between recent and persistent QFT+ individuals with the highest accuracy (39). With a simplified assay, we then tested the T-cell activation biomarker in a blinded test cohort of recent (n = 25) and persistent (n = 25) QFT+ adolescents and adults with TB disease (n = 25) (Figures 1A and 1B).
In the training cohort, we measured the expression of HLA-DR, IFN-γ, and TNF in live CD3+ T cells upon PBMC stimulation with CFP-10/ESAT-6 (Figures 2A and E2A and E3A). IFN-γ concentrations measured by QFT and flow cytometry were strongly correlated (Figure E3B). Activation was measured on cytokine-producing T cells as HLA-DR MFI; thus, the contribution of nonspecific signal from the unstimulated control cannot be subtracted. We defined antigen-specific T cells as IFN-γ+ TNF+ CD3+ cells, on the basis of previous observations that activation measured on CFP-10/ESAT-6–specific IFN-γ+ TNF+ could yield better diagnostic accuracy than IFN-γ+ T cells (20). The IFN-γ+ TNF+ subset was also associated with lower responses than IFN-γ+ CD3 T cells in unstimulated control subjects (Figure E3A).
Figure 3.
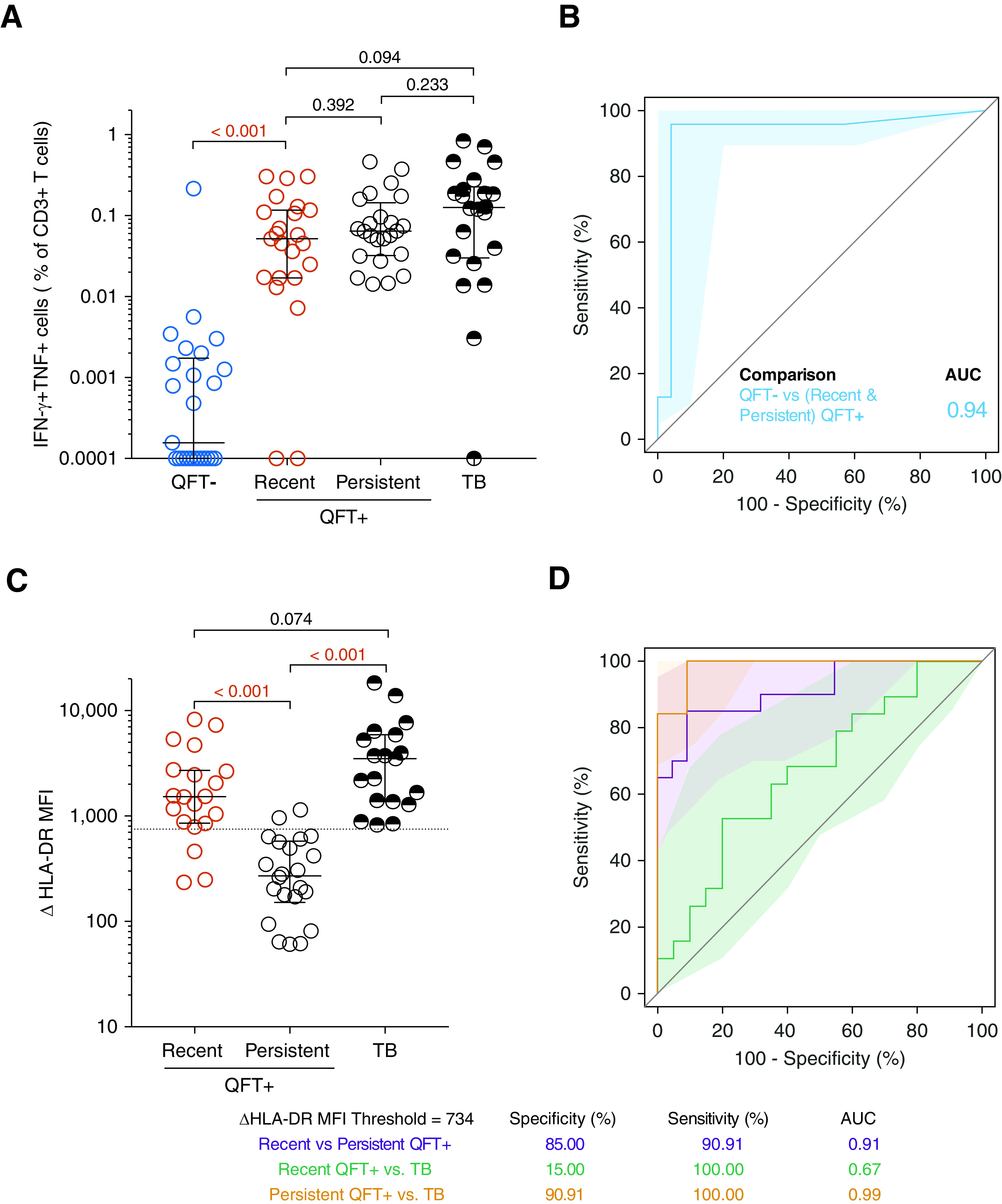
T-cell activation biomarker can distinguish between recent and persistent QuantiFERON-TB Gold (QFT)+ individuals as well as between persistent QFT+ individuals and individuals with tuberculosis (TB). (A) Frequencies of background subtracted CFP-10/ESAT-6–specific IFN-γ+ TNF+ CD3+ T cells detected before (QFT−; blue; n = 25) and after (recent QFT+; red; n = 23) QFT conversion, during persistent QFT+ (black open symbols, n = 24), and in patients with TB (black half-filled symbols; n = 22) in the test cohort. (B) Area under the receiver operating characteristic curve (AUROC) analysis illustrating the performance of CFP-10/ESAT-6–specific IFN-γ+ TNF+ CD3+ T cells to distinguish between samples taken before (QFT−) and after QFT conversion (recent or persistent QFT+ combined). (C) ΔHLA-DR median fluorescent intensity (MFI) in responders with recent QFT+ conversion (n = 20), persistent QFT+ results (n = 22), or TB (n = 19). (D) AUROC analysis depicting the performance of ΔHLA-DR MFI to discriminate between recent and persistent QFT+, between recent QFT+ and TB and between persistent QFT+ and TB. P values were calculated using the Wilcoxon signed-rank test for paired (QFT− vs. recent QFT+) or the Mann-Whitney U test for unpaired (all other) comparisons. Where appropriate, we corrected for multiple comparisons as described in the Methods. P values highlighted in red are considered significant. Shaded areas in AUROC plots depict 95% confidence intervals. Values <0.0001 were set to 0.0001 to allow display on a logarithmic scale. AUC = area under the curve.
QFT conversion was associated with an increase of CFP-10/ESAT-6–specific IFN-γ+ TNF+ and IFN-γ+ CD3 T cells, which were detectable at comparable frequencies in recent and persistent QFT+ individuals (Figures 2B and E3C). Similar to the QFT assay, frequencies of CFP-10/ESAT-6–specific IFN-γ+ TNF+ CD3 T cells allowed discrimination between QFT− and recent QFT+, with an area under the ROC curve (AUROC) of 0.89 (95% confidence interval [CI], 0.79–0.99), but not recent and persistent QFT+ (AUROC, 0.54; 95% CI, 0.38–0.70, Figure 2C). Results for IFN-γ+ CD3 T cells were similar (Figures E3C and E3D).
Analysis of T-cell activation in CFP-10/ESAT-6–stimulated samples (Figure 2D) was restricted to samples that passed our responder criteria (see Methods). ΔHLA-DR MFI (Figure 2E), proportions of HLA-DR+ IFN-γ+ TNF+ CD3+ T cells (Figure E4A), and HLA-DR MFI ratio (Figure E4B) were significantly higher in recent QFT+ individuals compared with persistent QFT+ individuals. All biomarkers yielded promising discriminatory potential with AUROCs of 0.91 (95% CI, 0.81–1.00) for ΔHLA-DR MFI (Figure 2F), 0.93 (95% CI, 0.84–1.00) for percentage HLA-DR, and 0.91 (95% CI, 0.80–1.00) for HLA-DR MFI ratio (Figure E4D). We also evaluated a published candidate biomarker for recent M.tb infection, namely proportions of TNF only–expressing effector (CD45RA−CCR7−) CD4+ T cells (27), and found similar concentrations in recent and persistent QFT+ individuals, with poor diagnostic performance (AUROC, 0.61; 95% CI, 0.42–0.80; Figures E4C and E4D). We selected ΔHLA-DR MFI for blinded confirmation in the test cohort because it does not require the selection of a discrete HLA-DR–positive cell subset using a threshold or “gate,” thus reducing operator bias, and also avoids the issue of negative MFI values that can result from flow cytometric compensation, which would affect calculation of the ratio-based biomarker.
Figure 4.
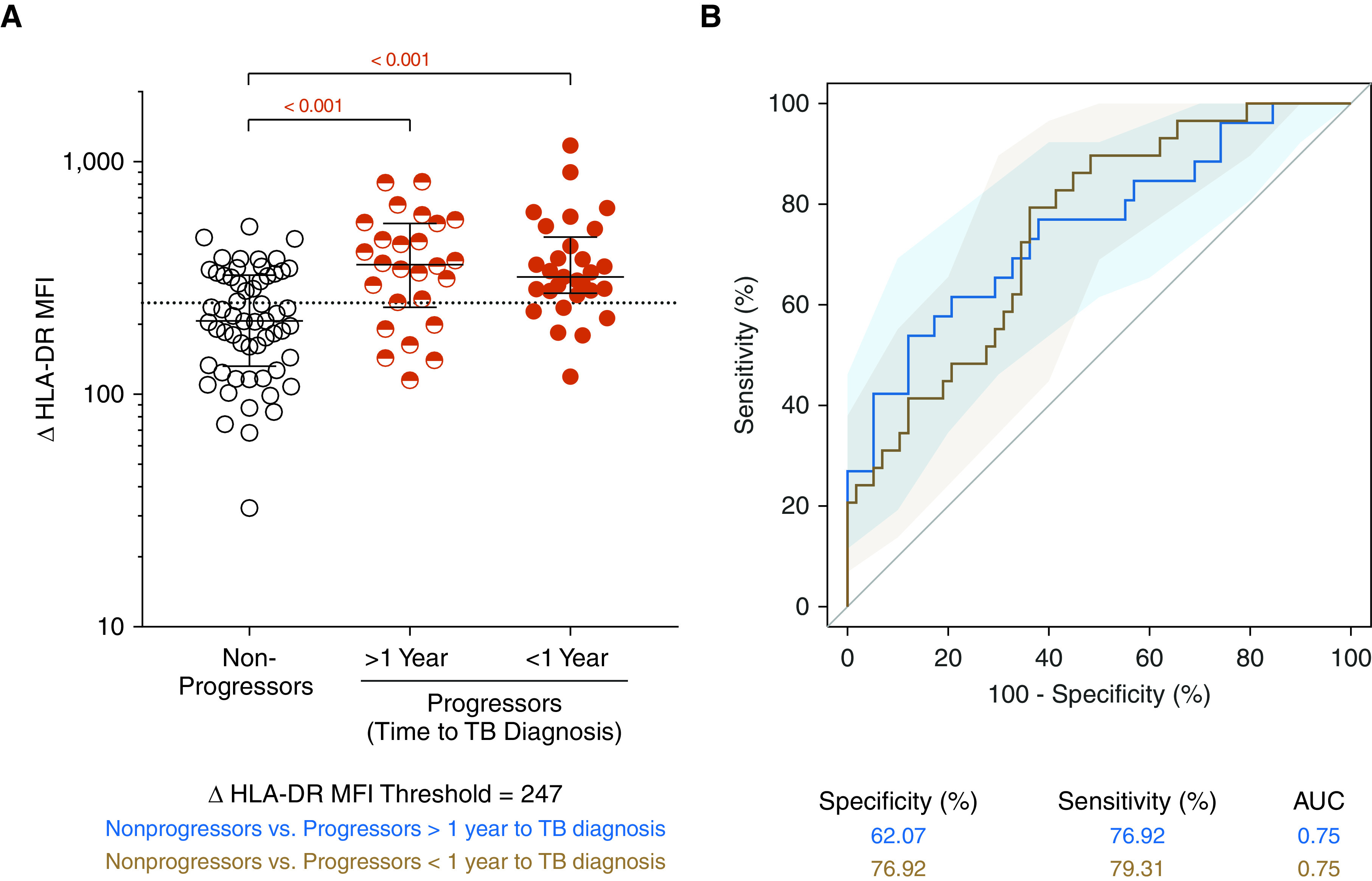
Mycobacteria-specific T cells in tuberculosis (TB) progressors are more activated than those in nonprogressors. (A) ΔHLA-DR median fluorescent intensity (MFI) on Mycobacterium tuberculosis lysate–responsive T cells coexpressing IFNG and TNF mRNA transcripts in nonprogressors (n = 58 longitudinal data points from 34 participants) and progressors (in samples collected >1 yr before TB diagnosis [>1 yr, n = 26 longitudinal data points from 19 participants] or in samples collected within 1 yr of tuberculosis diagnosis [<1 yr, n = 29 longitudinal data points from 22 participants]). P values were computed by Mann-Whitney U test and were corrected for two comparisons as described in the Methods. (B) Area under the receiver operating characteristic curve depicting the performance of ΔHLA-DR MFI to discriminate between nonprogressors and progressors in samples collected >1 year before TB or within 1 year of TB diagnosis. AUC = area under the curve.
Next, we evaluated the performance of the ΔHLA-DR MFI biomarker to distinguish between recent and persistent QFT+, recent QFT+ and TB, and persistent QFT+ and TB in the test cohorts. We developed a simplified version of the PBMC-ICS assay and an algorithm to provide a framework for interpreting test results as a biomarker for potential M.tb infection states (Table 2). M.tb infection was defined as a positive CFP-10/ESAT-6–specific IFN-γ+ TNF+ CD3+ T-cell response. In those with M.tb infection, individuals were then classified as those with persistent QFT+ and those with recent QFT+ or TB on the basis of their ΔHLA-DR MFI.
Table 2.
Interpretation of the T-Cell Activation Biomarker Test Results
|
M.tb-Specific Response Definition |
Responder Definition |
Recent M.tb Infection or TB Definition |
|||
|---|---|---|---|---|---|
| IFN-γ+ TNF+ T Cells ESAT-6/CFP-10 vs. UNS (Fisher Exact Test) | IFN-γ+ TNF+ T Cells PHA vs. UNS (Fisher Exact Test) | Interpretation | IFN-γ+ TNF+ T Cells ESAT-6/CFP-10 over UNS (Ratio) | Δ HLA-DR MFI (IFN-γ+ TNF+) − (Total T Cells) | Interpretation |
| P ≤ 0.01 | Any | M.tb infection likely | Threefold or greater | − (<734) | Persistent M.tb infection likely |
| + (≥734) | Recent M.tb infection or TB likely | ||||
| Less than threefold | N/A | N/A | |||
| P > 0.01 | P < 0.01 | M.tb infection unlikely | N/A | N/A | N/A |
| P > 0.01 | P > 0.01 | Indeterminate | N/A | N/A | N/A |
Definition of abbreviations: MFI = median fluorescent intensity; M.tb = Mycobacterium tuberculosis; N/A = not applicable; PHA = phytohemagglutinin positive control; TB = tuberculosis; UNS = unstimulated control.
CFP-10/ESAT-6–specific IFN-γ+ TNF+ CD3+ T cells induced by recent QFT conversion in the test cohort were detected at similar frequencies to those with persistent QFT+ reactivity or with TB (Figure 3A). Importantly, we were able to discriminate QFT– individuals from those with recent or persistent QFT+ with an AUROC of 0.94 (95% CI, 0.87–1.00; Figure 3B), suggesting that this simple flow cytometry–based assay yields equivalent diagnostic information to QFT.
ΔHLA-DR MFI was significantly higher in patients with recent QFT+ and TB compared with persistent QFT+ individuals (Figure 3C), however, individuals with recent QFT+ or TB disease showed similar ΔHLA-DR MFI. Using a ΔHLA-DR MFI cutoff of 734, we were able to discriminate between recent and persistent QFT+ with a specificity, sensitivity, and AUROC of 90%, 85%, and 0.91 (95% CI, 0.83–1.00), respectively. We also observed excellent discrimination between persistent QFT+ and TB, with high specificity (91%), sensitivity (100%), and AUROC (0.99; 95% CI, 0.96–1.00). ΔHLA-DR MFI could not accurately discriminate between recent QFT+ and TB (AUC, 0.67; 95% CI, 0.50–0.84; Figures 3C and 3D). Table E5 summarizes the outcomes of the diagnostic algorithm described in Table 2 when applied to the test cohort.
Our group previously reported a six-gene blood transcriptomic signature, RISK6, that can identify QFT+ and/or TST+ individuals who are at risk of progressing to TB (40). We therefore tested the ability of the ΔHLA-DR MFI biomarker to distinguish between progressors (n = 34) and nonprogressors (n = 34) in a longitudinal cohort of healthy QFT+ and/or TST+ adolescents (Figure 1C). Levels of ΔHLA-DR MFI were significantly higher in progressors up to 2 years before TB diagnosis compared with nonprogressors (AUROC, 0.75; 95% CI, 0.63–0.87; Figure 4). These results were consistent with the performance of RISK6 and suggest that the ΔHLA-DR MFI biomarker may also be useful for identifying individuals at risk of disease progression.
Discussion
We describe a simple biomarker, ΔHLA-DR MFI, that measures M.tb-specific T-cell frequencies and their activation levels, can distinguish QFT− (presumably uninfected) individuals from QFT+ (presumably infected) individuals, and, among the latter, can discriminate between those with recent QFT conversion, disease progression, or active TB and persistent QFT+ nonprogressors. This is a significant advance over current M.tb immunodiagnostics (TST and IGRA), which are unable to distinguish between different stages of M.tb infection and identify individuals at risk of progression to disease. Consistent with this limitation, we demonstrated that measuring M.tb-specific T-cell functions by flow cytometry can distinguish between QFT− and QFT+ individuals but not between QFT+ individuals and individuals with TB or recent and remote QFT conversion. Because individuals with recent QFT conversion and high risk for disease progression would benefit from targeted TPT, this biomarker offers an opportunity to identify a group of asymptomatic individuals at particularly high risk of TB, whereas those with persistent QFT+ test and low risk of progression would derive less benefit from TPT. This is of particular importance in high incidence settings, where a large proportion of the population is already M.tb infected and identification of individuals at high risk of TB progression is challenging. Our findings build on previous studies that have shown that measurement of M.tb-specific T-cell activation by flow cytometry has promising diagnostic potential to distinguish M.tb infection from TB disease, regardless of HIV status, as well as to monitor treatment response (16–20).
The immunological speculation that underpins our observations is that peripheral blood T-cell activation is driven by in vivo exposure to M.tb antigens and that these concentrations peak during primary infection and then drop in most people with remote infection, likely because of containment of bacterial replication. However, in people who progress toward active TB, in vivo M.tb antigen exposure is likely high even before the onset of clinical symptoms. Our measurements of T-cell activation during the different stages of infection provide evidence that supports this hypothesis. Specifically, we showed that a recent M.tb exposure (initial peak in M.tb burden) as well as a high bacterial load in progressors (before symptom onset) are associated with much higher levels of T-cell activation than those observed during presumed containment of M.tb replication (persistent QFT+ and nonprogressors). Furthermore, our findings suggest that remote infection is associated with low and stable amounts of M.tb-specific T-cell activation, consistent with control or perhaps even clearance of M.tb. This supports the hypothesis promulgated recently by Behr and colleagues that TB has a short incubation period (<2 yr) and that M.tb-specific T-cell responses detected with IGRAs in most individuals with remote M.tb exposure represent immunological memory, not ongoing M.tb replication (13). Indeed, a minority of individuals with remote M.tb exposure may harbor replicating M.tb and are at high risk of TB progression, as suggested by detection of high T-cell activation in progressors compared with nonprogressors. Therefore, our biomarker has utility to distinguish the majority of individuals with persistent QFT+ results and low risk of TB progression, who would be spared TPT, from the minority who harbor replicating M.tb, are at high risk of progression, and would benefit from further clinical investigation and TPT. Our inability to distinguish between recent QFT conversion and disease using the ΔHLA-DR MFI biomarker further supports the utility of T-cell activation as an indirect marker for M.tb antigen load, which is supposedly high in both these TB stages. To the best of our knowledge, whether TPT-mediated reduction of bacterial load would result in decreased HLA-DR expression on M.tb-specific T cells has not yet been shown; however, successful TB treatment is associated with a decrease in T-cell activation (16, 19, 20). We acknowledge that antibiotic regimens used for TPT and full course of treatment may differ and may have distinct impact on T-cell activation kinetics, and this should be addressed in future studies.
The strengths of our study include unique and well-characterized clinical cohorts, blinded verification of our findings in a test cohort, and translation of biological observations made with complex technology to a simple test that can be used for further validation.
Limitations of our study include convenience selection of participants on the basis of sample availability, which could have introduced a bias that would have been avoided by random selection. However, the finding that biomarker performance was reproduced in two cohorts (training and test) suggests that the effects of this bias are not significant. We were unable to formally validate our ΔHLA-DR MFI biomarker because the test, training, and progressor cohorts were selected from the same epidemiological study and were thus not completely independent (10). The control group of individuals with newly diagnosed TB included adults aged 21–42 years; therefore, we cannot exclude age-related differences. Furthermore, experimental protocols, stimulants, and reagents used to detect T-cell activation were different between cohorts, thus preventing us from using the same assay and defining a ΔHLA-DR MFI threshold that could be uniformly applied to all three cohorts. However, the consistency of results points toward robustness of the biomarker. Our cohort design did not include M.tb-uninfected individuals from low TB burden countries, nor do we have access to samples from such individuals; thus, we were unable to test the false positivity rate of our proposed biomarker in M.tb-unexposed individuals. We did, however, test the biomarker in individuals before QFT conversion and reported that only 1 of 25 QFT− participants was classified as likely infected.
The candidate biomarker proposed by Halliday and colleagues (TNF only–expressing T effector cells) (27) did not discriminate between recent and remote QFT conversion in our study. Compared with the original biomarker description, we used a different flow cytometry panel that lacked CD127, and therefore the cell subset definition was not identical.
Finally, experimental protocols described here were performed on cryopreserved PBMCs, a sample type that is not be ideal for clinical purposes but was the only one available for this retrospective study. Although this is not a point-of-care assay, we have shown that it can be adapted to a whole blood assay (such as QFT) with a simple four-color flow cytometry panel as readout (20), using basic technology that is widespread even in resource-limited settings and is suitable for automated analysis. It will be necessary to validate this simplified assay in large prospective field studies to estimate its true performance characteristics. Preferred performance characteristics of a test that can differentiate between recent and remote M.tb infection are currently not defined and would need to be developed to provide benchmarks for test performance. A validation is currently underway in a prospective South African pediatric cohort of household contacts of patients with TB.
In summary, we describe a blood-based T-cell biomarker, ΔHLA-DR MFI, measured with a simple flow cytometry assay, that can be used to identify individuals with recent QFT conversion and those with TB progression as well as clinical disease. Upon further validation, the ΔHLA-DR MFI biomarker has the potential to stratify individuals along the M.tb spectrum and could be considered as a screening test to identify individuals at high risk of TB for further evaluation. If interpreted in association with clinical features and microbiological tests, this biomarker could identify individuals with TB who would benefit from standard treatment, those at high risk who should receive TPT, and those who would not currently benefit from TPT but may be retested in the future.
Acknowledgments
Acknowledgment
The authors thank the study participants and their families; the Cape Winelands East district communities, the Department of Education and the Department of Health; the South African Tuberculosis Vaccine Initiative (SATVI) clinical and laboratory teams; Thomas Hawn and Jason Andrews for valuable input on the study design.
ACS Study Team: Hassan Mahomed, Willem A. Hanekom, Fazlin Kafaar, Leslie Workman, Humphrey Mulenga, Rodney Ehrlich, Mzwandile Erasmus, Deborah Abrahams, Anthony Hawkridge, E. Jane Hughes, Sizulu Moyo, Sebastian Gelderbloem, Michele Tameris, Hennie Geldenhuys, and Gregory Hussey.
Footnotes
A complete list of the ACS Study Team may be found before the beginning of the References.
Supported by the U.S. NIH (R21AI127121), Bill and Melinda Gates Foundation (OPP1066265, OPP1113682, GC 6–74 Grant 37772, and GC12 Grant 37885), Aeras, and the Strategic Health Innovation Partnerships Unit of the South African Medical Research Council with funds received from the South African Department of Science and Technology. Carnegie Corporation of New York funded scholarships to M.M.; South African National Research Foundation and the University of Cape Town funded C.A.M.M. The funding sources had no role in study design, data collection, analysis and interpretation, writing, and submission of the manuscript.
Author Contributions: M.M.D., M.R., M.H., T.J.S., and E.N. designed the study and raised funding. C.A.M.M., M.M., B.M., T.D.R., C.S., N.B., H.H., and G.O. generated the data. C.A.M.M., M.M., V.R., T.L., and H.H. analyzed the data. C.A.M.M., M.M., V.R., M.M.D., M.R., M.H., T.J.S., and E.N. interpreted the results. C.A.M.M., M.M., V.R., T.J.S., and E.N. wrote the manuscript. All authors revised and approved the manuscript and are accountable for the work.
Data are available on the zivahub UCT platform (https://zivahub.uct.ac.za), DOI: 10.25375/uct.14761596.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202007-2686OC on January 6, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: Hassan Mahomed, Willem A. Hanekom, Fazlin Kafaar, Leslie Workman, Humphrey Mulenga, Rodney Ehrlich, Mzwandile Erasmus, Deborah Abrahams, Anthony Hawkridge, E. Jane Hughes, Sizulu Moyo, Sebastian Gelderbloem, Michele Tameris, Hennie Geldenhuys, and Gregory Hussey
References
- 1.World Health Organization 2020https://www.who.int/tb/publications/global_report/en/.
- 2.World Health Organization 2020https://www.who.int/publications-detail/who-consolidated-guidelines-on-tuberculosis-module-1-prevention-tuberculosis-preventive-treatment.
- 3. Erkens CGM, Slump E, Verhagen M, Schimmel H, Cobelens F, van den Hof S. Risk of developing tuberculosis disease among persons diagnosed with latent tuberculosis infection in the Netherlands. Eur Respir J. 2016;48:1420–1428. doi: 10.1183/13993003.01157-2016. [DOI] [PubMed] [Google Scholar]
- 4. Reichler MR, Khan A, Sterling TR, Zhao H, Chen B, Yuan Y, et al. Tuberculosis Epidemiologic Studies Consortium Task Order 2 Team. Risk factors for tuberculosis and effect of preventive therapy among close contacts of persons with infectious tuberculosis. Clin Infect Dis. 2020;70:1562–1572. doi: 10.1093/cid/ciz438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019;54:1900655. doi: 10.1183/13993003.00655-2019. [DOI] [PubMed] [Google Scholar]
- 6. Mathema B, Andrews JR, Cohen T, Borgdorff MW, Behr M, Glynn JR, et al. Drivers of tuberculosis transmission. J Infect Dis. 2017;216:S644–S653. doi: 10.1093/infdis/jix354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Churchyard G, Kim P, Shah NS, Rustomjee R, Gandhi N, Mathema B, et al. What we know about tuberculosis transmission: an overview. J Infect Dis. 2017;216:S629–S635. doi: 10.1093/infdis/jix362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiker HG, Mustafa T, Bjune GA, Harboe M. Evidence for waning of latency in a cohort study of tuberculosis. BMC Infect Dis. 2010;10:37. doi: 10.1186/1471-2334-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ. 2018;362:k2738. doi: 10.1136/bmj.k2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Machingaidze S, Verver S, Mulenga H, Abrahams D-A, Hatherill M, Hanekom W, et al. Predictive value of recent QuantiFERON conversion for tuberculosis disease in adolescents. Am J Respir Crit Care Med. 2012;186:1051–1056. doi: 10.1164/rccm.201206-1134OC. [DOI] [PubMed] [Google Scholar]
- 11. Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis. 2012;54:784–791. doi: 10.1093/cid/cir951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cadena AM, Hopkins FF, Maiello P, Carey AF, Wong EA, Martin CJ, et al. Concurrent infection with Mycobacterium tuberculosis confers robust protection against secondary infection in macaques. PLoS Pathog. 2018;14:e1007305. doi: 10.1371/journal.ppat.1007305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Behr MA, Edelstein PH, Ramakrishnan L. Is Mycobacterium tuberculosis infection life long? BMJ. 2019;367:l5770. doi: 10.1136/bmj.l5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization 2017https://apps.who.int/iris/handle/10665/259176.
- 15.STOP TB Partnership http://www.stoptb.org/assets/documents/global/plan/GPR_2018-2022_Digital.pdf.
- 16. Riou C, Du Bruyn E, Ruzive S, Goliath RT, Lindestam Arlehamn CS, Sette A, et al. Disease extent and anti-tubercular treatment response correlates with Mycobacterium tuberculosis-specific CD4 T-cell phenotype regardless of HIV-1 status. Clin Transl Immunology. 2020;9:e1176. doi: 10.1002/cti2.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Riou C, Berkowitz N, Goliath R, Burgers WA, Wilkinson RJ. Analysis of the phenotype of Mycobacterium tuberculosis-specific CD4+ T cells to discriminate latent from active tuberculosis in HIV-uninfected and HIV-infected individuals. Front Immunol. 2017;8:968. doi: 10.3389/fimmu.2017.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilkinson KA, Oni T, Gideon HP, Goliath R, Wilkinson RJ, Riou C. Activation profile of Mycobacterium tuberculosis-specific CD4(+) T cells reflects disease activity irrespective of HIV status. Am J Respir Crit Care Med. 2016;193:1307–1310. doi: 10.1164/rccm.201601-0116LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Adekambi T, Ibegbu CC, Cagle S, Kalokhe AS, Wang YF, Hu Y, et al. Biomarkers on patient T cells diagnose active tuberculosis and monitor treatment response. J Clin Invest. 2015;125:1827–1838. doi: 10.1172/JCI77990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Musvosvi M, Duffy D, Filander E, Africa H, Mabwe S, Jaxa L, et al. T-cell biomarkers for diagnosis of tuberculosis: candidate evaluation by a simple whole blood assay for clinical translation. Eur Respir J. 2018;51:1800153. doi: 10.1183/13993003.00153-2018. [DOI] [PubMed] [Google Scholar]
- 21. Portevin D, Moukambi F, Clowes P, Bauer A, Chachage M, Ntinginya NE, et al. Assessment of the novel T-cell activation marker-tuberculosis assay for diagnosis of active tuberculosis in children: a prospective proof-of-concept study. Lancet Infect Dis. 2014;14:931–938. doi: 10.1016/S1473-3099(14)70884-9. [DOI] [PubMed] [Google Scholar]
- 22. Petruccioli E, Petrone L, Vanini V, Cuzzi G, Navarra A, Gualano G, et al. Assessment of CD27 expression as a tool for active and latent tuberculosis diagnosis. J Infect. 2015;71:526–533. doi: 10.1016/j.jinf.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 23. Rozot V, Patrizia A, Vigano S, Mazza-Stalder J, Idrizi E, Day CL, et al. Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin Infect Dis. 2015;60:432–437. doi: 10.1093/cid/ciu795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harari A, Rozot V, Bellutti Enders F, Perreau M, Stalder JM, Nicod LP, et al. Dominant TNF-α+ Mycobacterium tuberculosis-specific CD4+ T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andersen P, Doherty TM, Pai M, Weldingh K. The prognosis of latent tuberculosis: can disease be predicted? Trends Mol Med. 2007;13:175–182. doi: 10.1016/j.molmed.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 26. Scriba TJ, Penn-Nicholson A, Shankar S, Hraha T, Thompson EG, Sterling D, et al. other members of the ACS cohort study team. Sequential inflammatory processes define human progression from M. tuberculosis infection to tuberculosis disease. PLoS Pathog. 2017;13:e1006687. doi: 10.1371/journal.ppat.1006687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Halliday A, Whitworth H, Kottoor SH, Niazi U, Menzies S, Kunst H, et al. Stratification of latent Mycobacterium tuberculosis infection by cellular immune profiling. J Infect Dis. 2017;215:1480–1487. doi: 10.1093/infdis/jix107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mpande CAM.2018.
- 29.Mpande CAM.2018.
- 30.Mpande CAM.2019.
- 31.Musvosvi M.
- 32.Nemes E.2020.
- 33.Nemes E.2020.
- 34. Nemes E, Rozot V, Geldenhuys H, Bilek N, Mabwe S, Abrahams D, et al. C-040-404 Study Team and the Adolescent Cohort Study Team. Optimization and Interpretation of Serial QuantiFERON testing to measure acquisition of Mycobacterium tuberculosis infection. Am J Respir Crit Care Med. 2017;196:638–648. doi: 10.1164/rccm.201704-0817OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zak DE, Penn-Nicholson A, Scriba TJ, Thompson E, Suliman S, Amon LM, et al. ACS and GC6-74 cohort study groups. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet. 2016;387:2312–2322. doi: 10.1016/S0140-6736(15)01316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han A, Glanville J, Hansmann L, Davis MM. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat Biotechnol. 2014;32:684–692. doi: 10.1038/nbt.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glanville J, Huang H, Nau A, Hatton O, Wagar LE, Rubelt F, et al. Identifying specificity groups in the T cell receptor repertoire. Nature. 2017;547:94–98. doi: 10.1038/nature22976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez J-C, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mpande CAM, Rozot V, Mosito B, Musvosvi M, Dintwe OB, Bilek N, et al. Immune profiling of Mycobacterium tuberculosis-specific T cells in recent and remote infection. Ebiomedicine. 2021;64:103233. doi: 10.1016/j.ebiom.2021.103233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Penn-Nicholson A, Mbandi SK, Thompson E, Mendelsohn SC, Suliman S, Chegou NN, et al. Adolescent Cohort Study team; GC6-74 Consortium; SATVI Clinical and Laboratory Team; ScreenTB Consortium; AE-TBC Consortium; RePORT Brazil Team; Peruvian Household Contacts Cohort Team; CAPRISA IMPRESS team. RISK6, a 6-gene transcriptomic signature of TB disease risk, diagnosis and treatment response. Sci Rep. 2020;10:8629. doi: 10.1038/s41598-020-65043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


