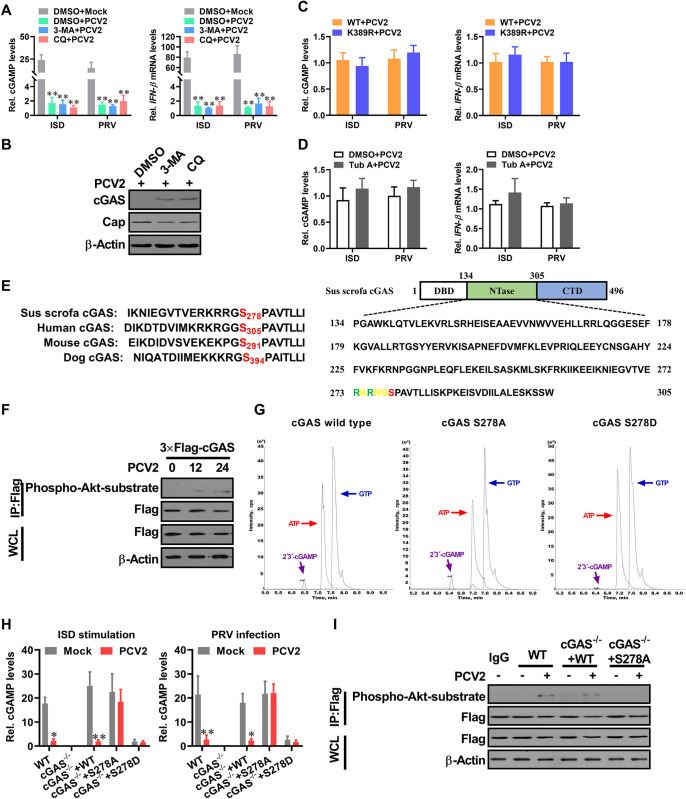
Fig 5. PCV2 induces phosphorylation of porcine cGAS at Ser278 to negatively regulate cGAS enzymatic activity.
(A, B) Suppression of autophagic flux cannot effectively improve the induction of cGAMP and IFN-β in PCV2-infected cells. PK-15 cells pretreated with 3-MA or CQ were infected with PCV2 (MOI = 5) for 48 h, and then the relative cGAMP production levels and IFN-β mRNA levels at 6 h following ISD stimulation or PRV infection were determined by report assay and qPCR respectively (A). ** P < 0.01 (compared with mock infection). The levels of porcine cGAS and PCV2 capsid were determined by western blotting (B). (C) The cGAS-/- PK-15 cells were reconstituted with the wild type cGAS, mutant cGAS (K389R), respectively, then infected with PCV2 (MOI = 5) for 48 h to detect the relative cGAMP and IFN-β mRNA production levels at 6 h following ISD stimulation or PRV infection. (D) PK-15 cells pretreated with Tub A (DMSO as control) were infected with PCV2 (MOI = 5) for 48 h, then the relative cGAMP and IFN-β mRNA production levels were determined at 6 h following ISD stimulation or PRV infection. (E) Sequence comparison of the cGAS phosphorylation site from the indicated species (upper panel). The potential phosphorylated peptides in the NTase motif of porcine cGAS are highlighted in green and red (lower panel). (F) PK-15 cells were infected with mock or PCV2 for indicated times, and the phosphorylation level of cGAS at the S278 site was detected by western blotting using a rabbit anti-Phospho-Akt Substrate monoclonal antibody that can recognize the motif (RRGS*278) of porcine cGAS. (G) The phosphorylation of porcine cGAS at Ser278 exhibits a weakened enzymatic activity in in vitro assay. LC-MS analysis of cGAMP production from an in vitro cGAMP synthesis assay. Small molecules were extracted from in vitro tubes which contained the same doses of wild-type cGAS, phosphorylation-resistant S278A mutant, and phosphomimetic S278D mutant for analysis of cGAMP isomers by tandem mass spectrometry. (H) The cGAS-/- PK-15 cells reconstituted with the WT cGAS, or cGAS mutant S278A, or cGAS mutant S278D were infected with mock or PCV2 in the presence of Baf for 12 h, and then the relative cGAMP production levels were determined at 6 h following ISD stimulation or PRV infection. * P < 0.05, ** P < 0.01 (compared with mock infection). (I) The cGAS-/- PK-15 cells reconstituted with the WT cGAS, or cGAS mutant S278A were infected with mock or PCV2 for 12 h, and then the phosphorylation level of cGAS at the S278 site was detected by Immunoprecipitation.