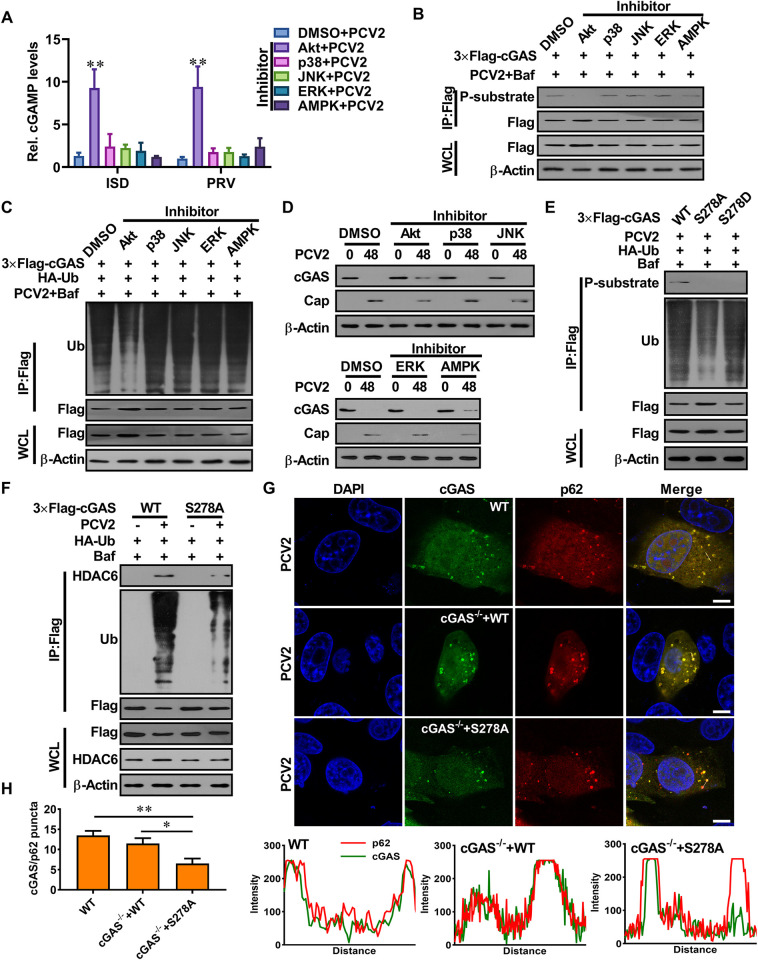
Fig 6. The phosphorylation of porcine cGAS at Ser278 facilitates the K48-linked poly-ubiquitination and degradation of porcine cGAS during PCV2 infection.
(A-B) Akt signaling negatively regulates cGAS-mediated cGAMP production in the PCV2-infected cells via phosphorylation of porcine cGAS at S278. PK-15 cells pretreated with indicated inhibitors were infected with PCV2 (MOI = 5) for 12 h, and then the relative cGAMP production levels were determined at 6 h following ISD stimulation or PRV infection (A); the phosphorylation level of cGAS at the S278 site was detected by western blotting (B). * P < 0.05, ** P < 0.01 (compared with DMSO/mock infection). (C, D) The phosphorylation of cGAS facilitates the ubiquitination and degradation of cGAS during PCV2 infection. PK-15 cells transfected with indicated expression constructs were treated with indicated inhibitors and then infected with PCV2 (MOI = 5) in the presence or absence of Baf to detect the poly-ubiquitination levels and protein levels of cGAS. (E) The cGAS-/- PK-15 cells were reconstituted with the WT cGAS, or S278A mutant, or S278D mutant, then infected with PCV2 (MOI = 5) to detect the poly-ubiquitination levels and phosphorylation levels of cGAS in these cells. (F) The cGAS-/- PK-15 cells were reconstituted with WT cGAS, or S278A mutant, then infected with PCV2 (MOI = 5) or mock to detect the interaction of ubiquitinated cGAS with HDAC6. (G) The wild-type PK-15 cells, and cGAS-/- PK-15 cells were reconstituted with WT cGAS or S278A mutant, then infected with PCV2 to observe the colocalization of cGAS and p62. The co-localization signals of targeted proteins were analyzed as intensity profiles of indicated proteins along the plotted lines by Image J line scan analysis. Scale bar, 10 μm.