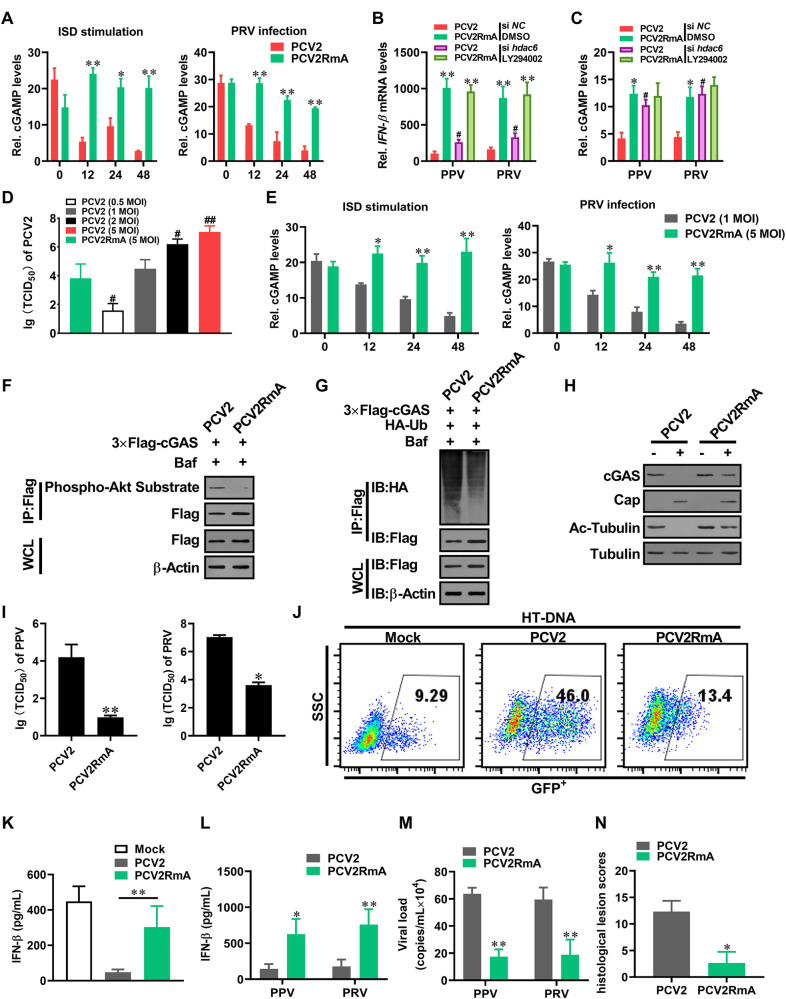
Fig 8. gC1qR-binding activity deficient PCV2 mutant (PCV2RmA) shows a weakened inhibitory effect on IFN-β induction and a weaker boost effect for other DNA viruses.
(A) PCV2RmA exhibits a lower inhibitory effect on other DNA-induced cGAMP compared with wild type PCV2. PK-15 cells were infected with wild type PCV2 (MOI = 5) or PCV2RmA (MOI = 5) for the indicated time, and then the relative cGAMP production levels were determined at 6 h following ISD stimulation or PRV infection. * P < 0.05, ** P < 0.01 (compared with PCV2 infection). (B, C) PK-15 cells were transfected with HDAC6 specific siRNA (siHDAC6) or control siRNA (siNC) for 24 h, then treated with Akt inhibitor or DMSO for 6 h, followed by PCV2 (MOI = 5) or PCV2RmA (MOI = 5) infection, and then the IFN-β mRNA levels or cGAMP production were determined at 6 h following PRV or PPV infection. * P < 0.05, ** P < 0.01 (compared with PCV2 infection in indicated same condition). # P < 0.05 (compared with the cells that transfected siNC and treated with DMSO in same infection). (D) PK-15 cells were infected with PCV2 at 0.5~5 MOI or PCV2RmA at 5 MOI, and progeny virion production was determined by TCID50 at 48 h post-infection. # P < 0.05, ## P < 0.01 (compared with 5 MOI PCV2RmA-infected PK-15 cells). (E) PK-15 cells were infected with wild type PCV2 (MOI = 1) or PCV2RmA (MOI = 5) for the indicated time, and then the relative cGAMP production levels and IFN-β mRNA levels were determined at 6 h following ISD stimulation or PRV and PPV infection. * P < 0.05, ** P < 0.01 (compared with PCV2 infection). (F-H) PCV2RmA exhibits a lower induction capability in the phosphorylation, poly-ubiquitination, and degradation of porcine cGAS compared with wild type PCV2. PK-15 cells were infected with wild type PCV2 (MOI = 1) or PCV2RmA (MOI = 5) for 12 h, the phosphorylation of cGAS was detected by immunoprecipitation (F), PK-15 cells were infected with wild type PCV2 (MOI = 1) or PCV2RmA (MOI = 5) for 48 h, then poly-ubiquitination (G), and protein levels of cGAS and Ac-tubulin levels (H) were detected by immunoprecipitation. (I-K) PCV2RmA is a weak strain relative to PCV2 in promotion of DNA virus replication. PK-15 cells were infected with PCV2 (MOI = 1) or PCV2RmA (MOI = 5) for 48 h, then were further infected with PPV or PRV, and the relative viral titers were measured by TCID50 (I). * P < 0.05, ** P < 0.01. (J) Comparison of VSV-GFP replication in PK-15 cells pretreated with cell supernatants from HT-DNA-stimulated mock-infected cells, HT-DNA-stimulated PCV2-infected cells, or HT-DNA-stimulated PCV2RmA-infected cells. GFP positive cells were measured by flow cytometry. (K) IFN-β levels were measured by ELISA. ** P < 0.01. (L-N) PCV2RmA alleviating PPV- or PRV-induced pathological changes. The piglets (n = 3 per group) were infected by PCV2 (4×105 TCID50), PCV2RmA (2×106 TCID50) for 1 week, respectively, and then challenged with 105 TCID50 PPV or 105 TCID50 PRV for another one week. (L) The serum IFN-β of the infected piglets were measured by ELISA at 24 h post-2nd infection. * P < 0.05, ** P < 0.01 (compared with PCV2 infected pigs). (M) The viral load of porcine parvovirus (PPV) or porcine pseudorabies virus (PRV) were compared between PCV2 infected pigs and PCV2RmA infected pigs by qPCR. The PPV and PRV loads in the serum of PCV2-infected pigs are higher than that in the serum of PCV2RmA-infected pigs. * P < 0.05, ** P < 0.01 (compared with PCV2 infected pigs). (N) The histological lesion scores of infection piglets (PCV2 and PCV2RmA).