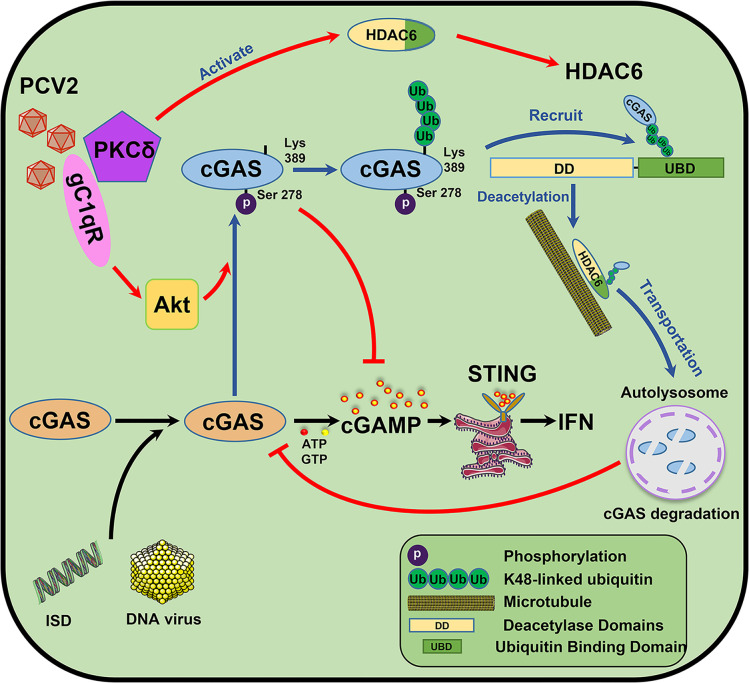
Fig 9. Model of PCV2 targeting cGAS to suppress Type I interferon production by gC1qR-mediated catalytic activity inhibition and HDAC6-mediated autophagic degradation.
In the early phase of PCV2-infection, porcine cGAS is phosphorylated at S278 site via Cap binding protein gC1qR-mediated PI3K/AKT signaling activation, which directly silences the catalytic activity of cGAS to inhibit cGAMP production; Subsequently, phosphorylation of cGAS at S278 facilitates the K48-linked poly-ubiquitination of cGAS at K389, while PKCδ is phosphorylated and recruited by gC1qR to promote HDAC6 activation; then K48-ubiquitinated-cGAS proteins are recruited and transported from the cytosol to autolysosome by activated histone deacetylase 6 (HDAC6) depending on its deacetylase activity and ubiquitin-binding function, thereby eventually resulting in a markedly increased cGAS degradation in PCV2 infection-induced autophagic cells.