Figure 9. High‐dose milk fat globule‐epidermal growth factor (MFG‐E8) treatment attenuates ligation‐induced β1 integrin and Rac1 activation and alleviates neointimal hyperplasia after vascular injury by limiting vascular smooth muscle cell (VSMC) migration.
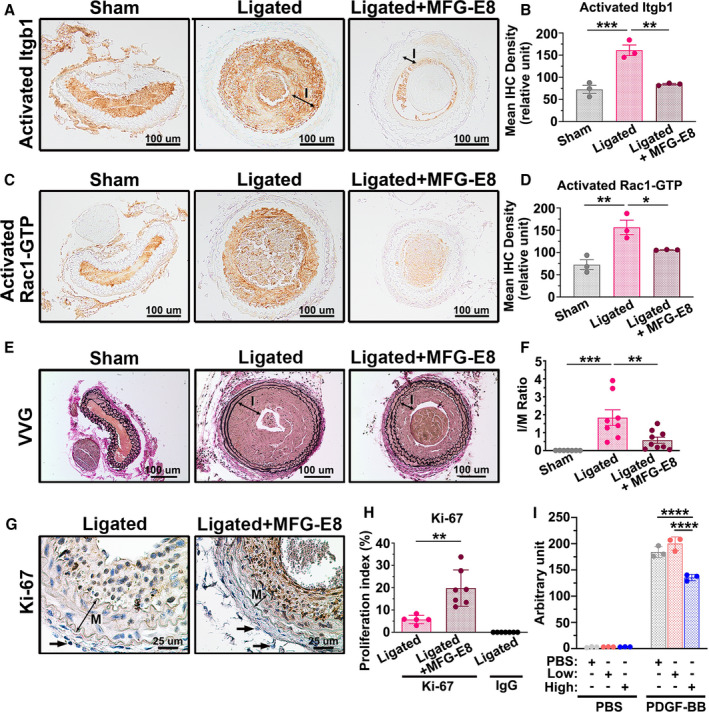
Recombinant MFG‐E8 (rMFG‐E8) (2 μg/mL) was delivered using pluronic gel into the carotid artery after complete ligation of the vessel. A, Representative immunohistochemistry images of activated β1 integrin (Itgb1) in sham‐operated common carotid arteries (CCAs), ligated CCAs, and ligated CCAs treated with high‐dose MFG‐E8 in wild‐type (WT) mice 21 days after ligation. Bar, 100 μm. B, Quantitative analysis of the immunostaining intensities of active β1 integrin in the intimal–medial area was performed (nmice=3 for each experimental group). Data are presented as mean±SEM. Each point is derived from an assessment of 3 sections of an individual animal. ** P<0.01 and ***P<0.001, as obtained using 1‐way ANOVA followed by Tukey multiple comparisons test. C, Representative immunohistochemistry photomicrographs of activated Rac1‐GTP in sham‐operated CCAs, ligated CCAs, and ligated CCAs treated with high‐dose MFG‐E8 in WT mice 21 days after ligation. Bar, 100 μm. D, Immunostaining intensity of the activated Rac1‐GTP in the intimal–medial area (nmice=3 for each experimental group) was quantified. Data are presented as mean±SEM. Each point is derived from an assessment of 3 sections of an individual animal. *P<0.05 and **P<0.01, as obtained using 1‐way ANOVA followed by Tukey multiple comparisons test. E, Representative images displaying the cross‐sectional areas of sham‐operated CCAs, ligated CCAs, and ligated CCAs treated with rMFG‐E8 in WT mice 21 days after ligation. Verhoeff–van Gieson (VVG) staining was performed on all sections. The intima (labeled I) in the ligated artery is indicated. Bar, 100 μm. F, Morphometric analysis of the intima/media (I/M) ratio was conducted 21 days after ligation (sham: nmice=7, ligated: nmice=8, ligated+MFG‐E8: nmice=9). Data are presented as mean±SEM. Each point is derived from an assessment of 3 sections of an individual animal. **P<0.01 and ***P<0.001, as obtained using 1‐way ANOVA followed by Tukey multiple comparisons test. G, Photomicrographs displaying immunostaining of Ki‐67 in cross sections of the ligated CCAs and ligated CCAs treated with rMFG‐E8 at 10 days after ligation. Negative control for the antibody in the intima–media of the vessel 10 days after ligation is shown in Figure S7. The media (labeled M) in the ligated artery is indicated. Bar, 25 μm. Arrows denote the fibroblasts in the adventitia. The similar sizes of the fibroblasts in ligated CCAs and MFG‐E8–treated CCAs affirm the same magnification in the 2 figures. H, Quantitative immunohistochemistry analysis of the proliferation index (Ki‐67[+] cells per total cell number) in the intima–media of the vessel 10 days after ligation (ligated: nmice=5, ligated+MFG‐E8: nmice=7, IgG: nmice=7). Data are presented as mean±SEM. Each point is derived from an assessment of 3 sections of an individual animal. **P<0.01, as obtained using the t test. I, A10 VSMCs were treated overnight with vehicle or various doses of rMFG‐E8. The cells were trypsinized and seeded on transwells with a pore size of 8 μm in 24‐well plates; thereafter, a total of 800 µL of PBS or platelet‐derived growth factor‐BB (PDGF‐BB) (10 ng/mL) was added to Dulbecco's Modified Eagle Medium (DMEM) in the lower chamber to induce VSMC migration. The A10 cells that migrated into the lower chambers were stained with calcein‐AM, and the fluorescence intensity was quantitated. Data represent the averages of triplicate samples of a representative experiment (n=3), and the error bars indicate the SD. ****P<0.0001, as obtained using 1‐way ANOVA followed by Tukey multiple comparisons test. IHC indicates immunohistochemistry; Itgb1, beta1 Integrin; and MFG‐E8, milk fat globule‐epidermal growth factor 8.
