Figure 1.
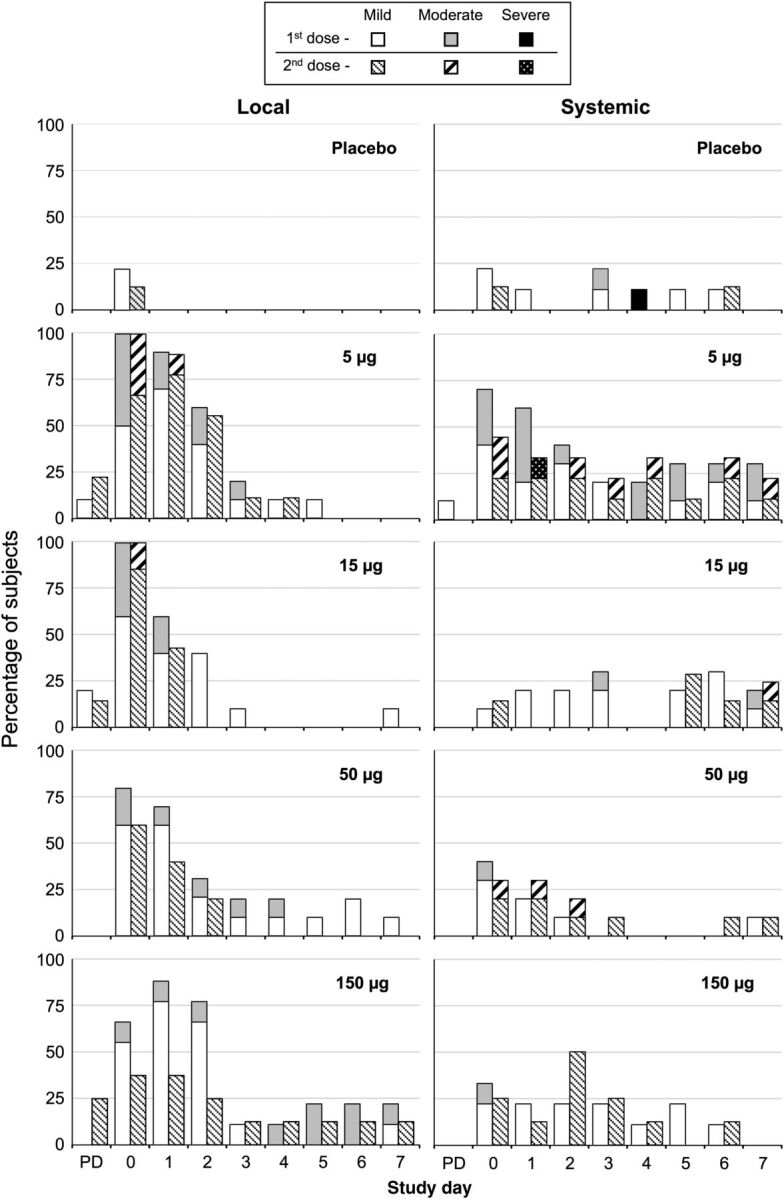
Dose-related reactogenicity of the bivalent norovirus virus-like particle vaccine formulated with aluminum hydroxide and MPL (3-O-desacyl-4′-monophosphoryl lipid A; GlaxoSmithKline) in 18–49 year olds (cohort A). Frequencies of local and systemic reactogenicity events are shown by day after doses 1 and 2, with maximum severity during the observation period assessed as mild, moderate, or severe. The most common local complaints were pain and tenderness at the injection site, and the most common systemic complaint was headache. Post dosing assessment, performed 30 minutes after dosing. Abbreviation: PD, post dosing assessment.
