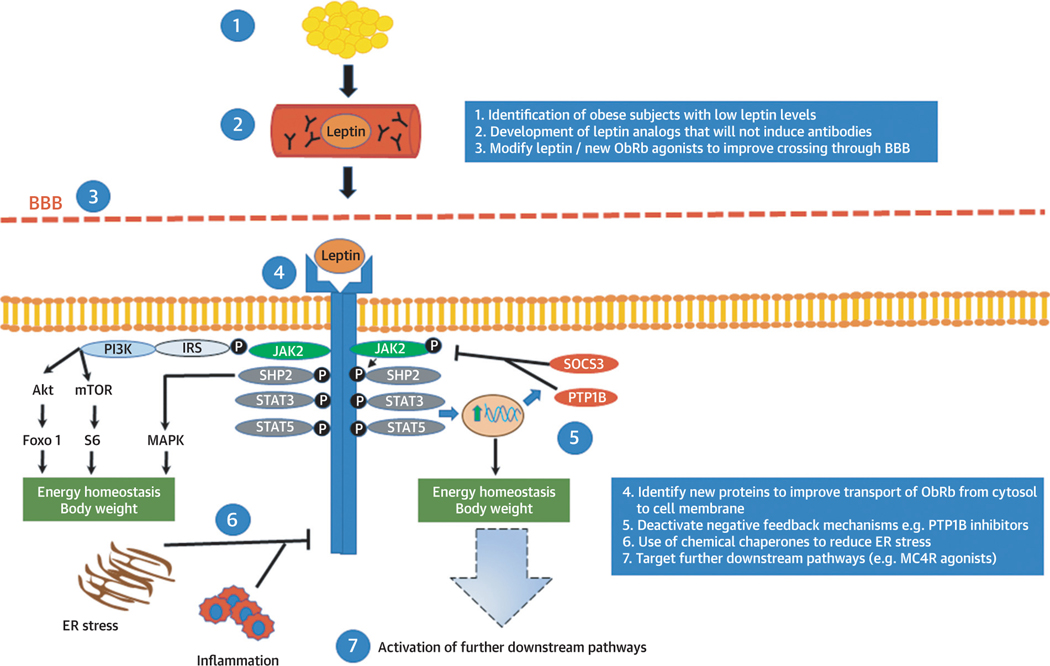
FIGURE 2.
Current Approaches Aiming to Improve the Efficiency of Leptin Treatments in Common Obesity
Leptin is secreted mainly by adipose tissue and circulates in the bloodstream crossing the blood-brain barrier (BBB) and acting in the brain. Leptin’s binding on its receptor activates signaling pathways that are related to energy homeostasis and body weight regulation in humans. In common obesity, leptin treatment does not induce changes in body weight, suggesting potentially the presence of leptin tolerance or resistance. Current efforts aim to increase the efficiency of leptin treatment by following several approaches, such as by selecting obese patients with low leptin levels and thus higher chances for response or by improving transport of leptin- based treatments in the brain or by targeting the downstream signaling pathways related to leptin. Akt = ■■■; ER = endoplasmic reticulum; Foxo1, Forkhead box protein O1; IRS = insulin receptor substrate; JAK2 = Janus kinase 2; MAPK = mitogen-activated protein kinase; MC4R, melanocortin 4 receptor; mTOR = mammalian target of rapamycin; ObRb = long form of the leptin receptor; PI3K = phosphoinositide 3-kinase; PTP1B = protein-tyrosine phosphatase 1B; S6 = substrate 6; SHP2 = Src homology 2 domain-containing phosphatase 2; SOCS3 = suppressor of cytokine signaling 3; STAT = signal transducer and activator of transcription.